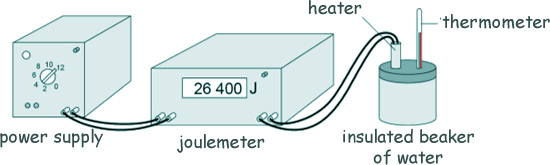
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q9. Andy carried out an experiment to determine the specific heat capacity of water. Here is a diagram of the equipment he used to heat the water.
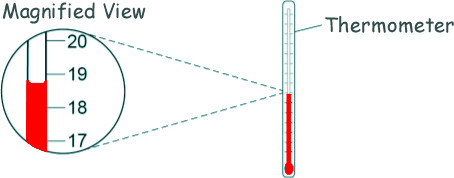
(a) Explain why Andy insulated the beaker of water. To prevent heat energy escaping to the surroundings. [1 mark] (b) One hazard in this experiment is the hot water. Give one risk caused by this hazard. Hot water could scald Andy. [1 mark] (c) The diagram shows of the thermometer that Andy used in more detail.
What is the resolution of the thermometer? ± 1 oC
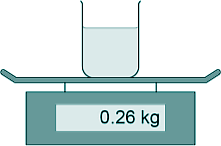
[1 mark] (d) The diagram shows the beaker of water on a balance.
The mass of the water was 200 g What was the mass of the beaker in kilograms? 200 g = 0.20 kg 0.26 - 0.20 = 0.06 kg [1 mark] (e) The energy transferred to the water was 26.4 kJ The mass of water was 0.20 kg. The temperature increase of the water was 30 °C. Calculate the specific heat capacity of water using the data from this experiment. Energy transferred = mass x SHC x temperature change E = mcΔT 26.4 = 0.20 x c x 30 c = 26.4/6 c = 4.4 OR c = 4400 [4 marks] (Total 8 marks) |
Follow me...
|