Specific Heat Capacity and Latent Heat Questions - GCSE standard
Q3. Buffy used the apparatus shown below to obtain the data needed to calculate the specific heat capacity of copper.
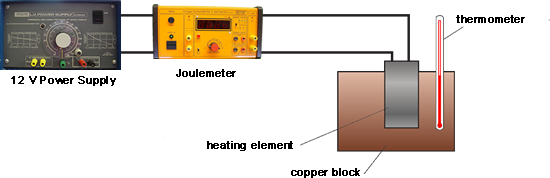
First she measured the initial temperature of the copper block. The power supply was then switched on.
Every minute, the energy transferred by the heater to the block was measured using the joulemeter and temperature of the block was taken and recorded. At the end of the experiment the temperature increase was calculated.
Buffy's results were entered into a table and then she plotted the graph below:
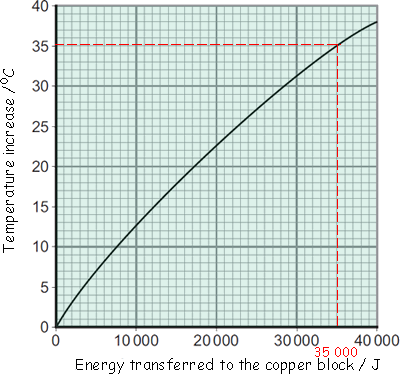
(a) Energy is transferred through the copper block. What is the name of the process by which the energy is transferred?
Conduction 
[1 mark]
(b) Use the graph to determine how much energy was needed to increase the temperature of the copper block by 35 °C.
(see graph) 35,000 J 
[1 mark]
(c) The copper block has a mass of 2 kg. Use your answer to part (b) to calculate the value given by this experiment for the specific heat capacity of copper. Remember to give the unit.
Energy supplied = mass x SHC x temperature rise
E = mc=ΔT
35 000 = 2 x SHC x 35
SHC = 35000/70  = 500
= 500 J kg-1 oC-1
J kg-1 oC-1
[3 marks]
(d) This experiment does not give the correct value for the specific heat of copper. Suggest one reason why.
The examiners wanted you to say that energy was lost to the surroundings. You could express that as energy would be needed to warm heater or energy would be lost to the room as there was no insulation (on the copper block). 
[1 mark]
(Total 6 Marks)








