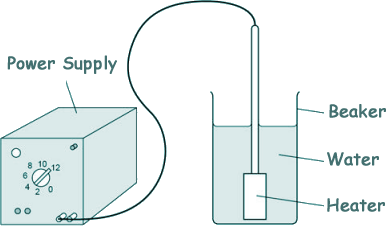
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q19. Mina determined the specific latent heat of vaporisation of water. The diagram shows some of the equipment used.
This is the method she used:
(a) What measuring instrument should be used to measure the volume of water? A measuring cylinder
[1 mark] (b) Tick one box to identify the hazard in the student's investigation.
[1 mark] (c) The initial mass of the beaker and water was 0.080 kg. The final mass of the beaker and water was 0.071 kg. The energy transferred by the immersion heater as the water boiled was 25 200 J. Calculate the specific latent heat of vaporisation of water given by Mina's data. energy = specific latent heat x mass E = LΔm L = E/Δm L = 25 200/(0.080 - 0.071) L = 25 200/0.009 L = 2 800 000 = 2.80 x 106 [5 marks] (d) Some thermal energy was transferred to the surroundings while the water was being heated. Explain how this affected the student's value for the specific latent heat of vaporisation of water. That thermal energy was not used to heat the water, therefore her value of the energy required was too high [2 marks] (e) Some of the water evaporated before its temperature reached 100 °C. Explain how this affected the student’s value for the specific latent heat of vaporisation of water. That evaporated water escaped before absorbing latent heat energy, therefore the mass loss of water will be exagerated [2 marks] (11 marks total) |
Follow me...
|