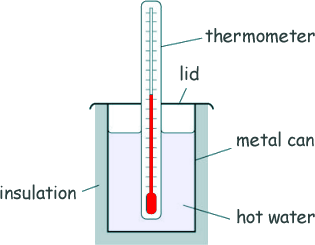
Specific Heat Capacity and Latent Heat Questions - GCSE standard Q18. Kayjay investigated the insulating properties of different materials. The diagram shows some of the equipment used by her.
This is the method she used:
(a) Identify the independent variable and the dependent variable in this investigation. The independent variable is the type of insulating material. The dependent variable is the time taken for the water to decrease by a fixed amount. [2 marks] (b) Kayjay used two different types of thermometer to measure the temperature changes. The diagram below shows a reading on each thermometer.
[1 mark]
[1 mark] (c) For one type of insulating material, the temperature of the water decreased from 85.0 °C to 65.0 °C. The energy transferred from the water was 10.5 kJ. Given that the specific heat capacity of water is 4200 J/kg °C, calculate the mass of water in the can. ΔT = 85.0 - 65.0 = 20.0 °C E = 10.5 kJ = 10 500 J c = 4200 J/kg °C E = mcΔT m = E/(cΔT) m = 10 500/(4200 x 20) m = 10 500/84 000 m = 0.125 kg [3 marks] (d) The table below shows the results for two insulating materials.
Explain how these results can be used to compare the thermal conductivity of the two materials. It takes less timefor material x to change temperature it is therefore conducting heat at a faster rate and is an better conductor than material X. [2 marks] (9 marks total) |
Follow me...
|