GCSE Questions: Medical Physics
- scanning and therapy
Q3.
(a) A radiation detector and counter were used to detect and measure the radiation emitted from a weak source. The graph shows how the number of counts recorded in one minute changed with time.
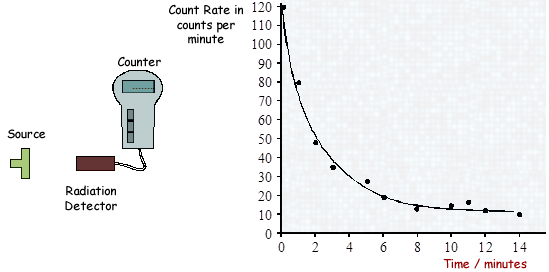
(i) Even though the readings from the counter were accurately recorded, not all the points fit the smooth curve.
What does this tell us about the process of radioactive decay?
It tells us that radioactive decay is random 
The examiner did not accept 'unpredictable' or 'irregular'.
(1 mark)
(ii) After ten minutes the number of counts recorded each minute is almost constant.
Explain why.
The source adds nothing or little to the count.  The detector is just recording background level.
The detector is just recording background level. 
(2 marks)
(b) The radioactive isotope sodium-24 injected into the bloodstream can be used to trace blood flow to the heart. Sodium-24 emits both beta particles and gamma rays.
(i) What is a beta particle?
A high energy electron that has been emitted from the nucleus. 
(1 mark)
(ii) What is a gamma ray?
High energy electromagnetic radiation with a high frequency (or short wavelength) 
(1 mark)
(iii) The count rate from a solution containing sodium-24 decreases from 584 counts per minute to 73 counts per minute in 45 hours.
Calculate the half-life of sodium-24.
Show clearly how you work out your answer.
584 to 292 in one half life
292 to 146 in the second half life
146 to 73 in the third half life 
So, 45 hours is three half lives 
One half life is therefore 45/3 = 15 hours 
(3 marks)
(iv) Give one advantage of using sodium-24 to trace blood flow compared to using an isotope with a half-life of:
[A] ten years
A safe level of radiation reached much quicker when you use the Na-24  - the person it was injected into would remain radioactive for too long for it to be safe to use.
- the person it was injected into would remain radioactive for too long for it to be safe to use.
(1 mark)
[B] ten seconds
Before you had a chance to carry out the diagnostic tests the ten second half life one would decay to background level. 
(1 mark)
(Total 10 marks)


