Multiple Choice: Materials, Energy, Heat and Temperature and Kinetic Theory Q1. These statements are about the pressure and volume of a fixed mass of gas at constant temperature. Which statement is correct?
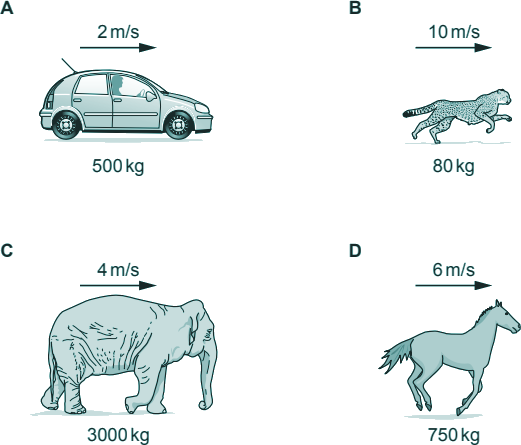
Q2. Which of the following has the most kinetic energy?
EK = ½mv2
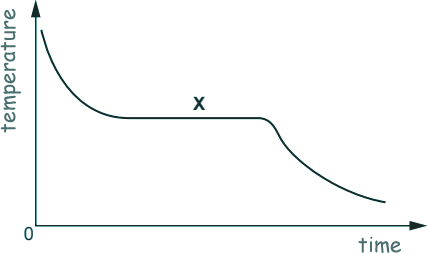
Q3. A student studies how the temperature falls when a liquid cools.
What is happening at point X on the graph?
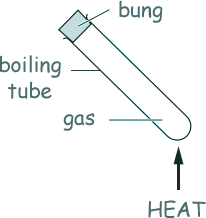
Q4. A sealed boiling tube contains gas. The boiling tube is heated.
What happens?
Q5. An object has a volume of 1.5 m3 and a mass of 3.0 kg. What is the density of the object? density = mass/volume density = 3.0 / 1.5 density = 2.0 kg/m3
Q6. A cylinder contains a gas. The volume of the gas is halved and the temperature remains the same. What happens to the pressure of the gas?
Q7. Different states of matter have different densities. Which of the following shows the states of matter in density order, starting with the lowest density?
Q8. What is the typical size for a small molecule?
Q9. Adeleine measures the weight of four boxes and the area in contact with the ground. Which box exerts the greatest pressure on the ground? Pressure = weight/volume
Q10. Energy is needed to change ice into water. Calculate the energy needed to change 5 kg of ice into water. Specific latent heat of melting = 3.34 × 105 J/kg. Energy to change state = specific latent heat x mass E = 3.34 × 105 x 5 = 1,670,000 J
|
Follow me...
|