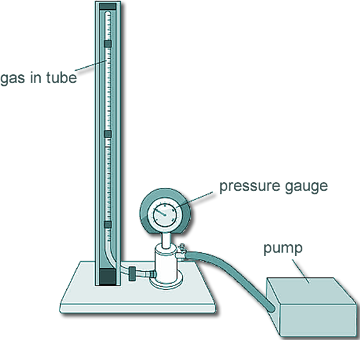
GCSE Questions: Kinetic Theory - Gases Q1. A student investigated how the pressure exerted by a gas varied with the volume of the gas. A pump was used to compress the gas in a tube. As the volume of the gas decreases, the pressure of the gas increases. The student used the pump to increase the pressure on the trapped mass of gas. Here is the equipment the student used:
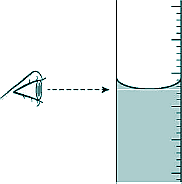
(a) The student only recorded one set of results. Give two reasons why taking repeat readings could provide more accurate data. [2 marks] (b) The diagram shows the position of the student's eye when taking volume measurements.
Explain what type of error would be caused if the student's eye was not in line with the level of the liquid in the tube. [2 marks] (c) If the gas is compressed too quickly the temperature of the gas increases. Explain how the temperature increase would affect the pressure exerted by the gas. [2 marks] (d) One of the student's results is given below: pressure = 1.6 × 105 Pa volume = 9.0 cm3 Calculate the volume of the gas when the pressure was 1.8 × 105 Pa. Assume the temperature of the gas was constant. [3 marks] (e) The photo shows a person using a bicycle pump to inflate a tyre.
The internal energy of the air increases as the tyre is inflated. Explain why. [2 marks] [11 Marks TOTAL] |
Follow me...
|