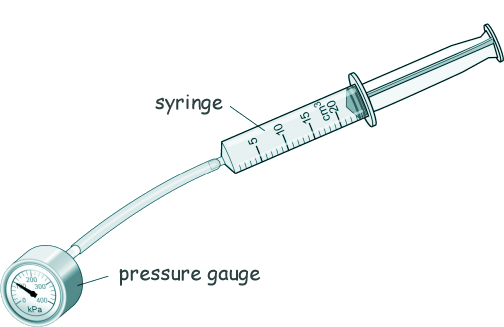
GCSE Questions: Kinetic Theory - Gases Q3. Ringo used the equipment shown in the diiagram to investigate how the pressure of a gas varies with the volume of the gas.
The syringe is filled with air. Here is a table of the results:
(a) Describe how Ringo could have used the equipment shown above to obtain the data shown in the table. [4 marks]
- the examiner looks for relevant points - but also for a logical sequence... It is not just a 'tick fest' - but a marker does look to see how many relevant points you have made, then considers how you have strung them together to put your answer into a 'level'
Indicative content:
(b) Describe what happens to the pressure of the air when the volume of the air is halved. When the volume is halved the pressure increases [2 marks] (c) The temperature of the air in the syringe remained constant during Ringo's investigation. Tick two properties of the air particles listed in the table below that would change if the temperature increased.
[2 marks] [8 Marks TOTAL] |
Follow me...
|