GCSE Questions: Kinetic Theory - Gases
Q2. Claire investigated the cooling effect of evaporation.
She used the equipment shown in the diagram to measure how the temperature of three different liquids changed as they evaporated.
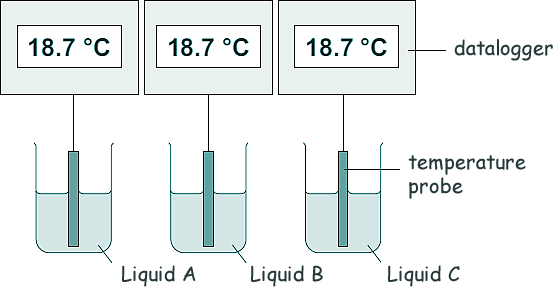
(a) The temperature and volume of each liquid was the same at the start of the investigation.
State one further control variable in this investigation.
Liquid surface area or shape of the beaker
OR
Duration of experiment 
[1 mark]
(b) Give two advantages of using dataloggers and temperature probes compared to using a thermometer as shown below.
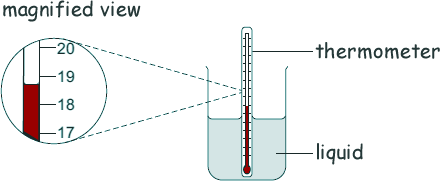
[2 marks]
Any two from: 

 takes readings automatically (do not say it makes it easier for you!)
takes readings automatically (do not say it makes it easier for you!)
 takes readings more frequently
takes readings more frequently
 reduces instrument reading error
reduces instrument reading error
 higher resolution
higher resolution
 don't need to remove probe to take reading
don't need to remove probe to take reading
 more accurate
more accurate
(c) Claire's results are plotted on the graph below:
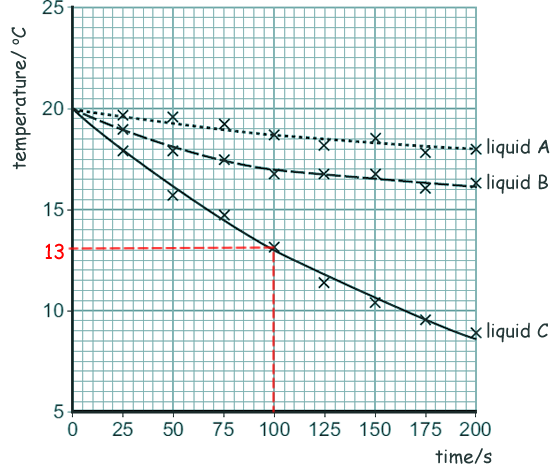
(i) Calculate the average rate of temperature decrease of liquid C between 0 and 100 seconds.
See graph
ΔT = 20oC - 13oC
ΔT = 7oC 
time = 100 s
Rate of temperature decrease = 7oC/100s
= 0.07  oC/s
oC/s
[3 marks]
(ii) Give one conclusion that can be made about the rate of temperature decrease of all three liquids from her results.
The rate of temperature change decreases as the experiment proceeds.
[1 mark]
(iii) Which liquid had the lowest rate of evaporation? Give a reason for your answer.
Liquid A - it has the
lowest temperature drop over 200 seconds. 
[1 mark]
(iv) Gabriel did the same investigation but using a smaller volume of liquid than Claire. All other variables were kept the same.
What effect would this have on the results of Gabriel's investigation?
There would be a larger temperature change per second - as the mass of liquid is lower but the rate of evaporation would be the same 
[1 mark]
(d) Explain how the evaporation of a liquid causes the temperature of the remaining liquid to decrease.
Temperature of a liquid is a measure of the average kinetic energy of the particles in it.Particles with higher than average kinetic energy leave the surface of the liquid
leave the surface of the liquid  during evaporations, which reduces the average kinetic energy of the remaining particles.
during evaporations, which reduces the average kinetic energy of the remaining particles. 
[3 marks]
[12 Marks TOTAL]









