| Misconception |
Discussion of the problem |
Possible activity |
Heat and temperature are the same
OR
Temperature is a measure of the heat content of a body |
Heat is energy (measured in J) Temperature is how hot or cold a body is - related to the kinetic energy of particles within the body - measured in K)
Change in heat content of a body depends on:
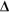 T - change in temperature T - change in temperature
m - mass
c - specific heat capacity
|
Practical: Give the same amount of heat energy (heat for the same time with a constant energy source) to different masses, volumes, types of substance... measure the change in temperature and discuss.
The SHC equationis not done until A level but can be done without use of the equation - just a knowledge of how the factors come into play. A worksheet with questions asking how doubleing the mass of water heated etc. affects the outcome can be useful. |
| Heat and cold are substances. |
They talk of heat or cold particles being passed from atom to atom or heat particles getting faster and slower. At KS3 they have not yet done wave-particle duality - so they are not thinking about photon transfer!
They have to think of heat as the energy that the particles gain or lose according to temperature difference... and cold as referring to temperature |
Worksheet with inaccurate use of terms for them to correct.
Could then be done on the whiteboard with class discussion as to why the sentences need correction. |
| Hot and cold temperatures are properties of materials. |
They think that metal objects are cold and poystyrene ones are hot (or at least warm) - when they are at room temperature.
That is because when they touch objects made of them at room temperature they feel hot or cold to them. The reason why metals feel cold is because they are good conductors - they take heat quickly away from the body - so the sensors in our fingertips relay the information to the brain that we are losing heat energy from our bodies moer quickly than before we touched the object.
The converse is true with polystyrene (or a 'warm' jumper). It is all down to heat transfer rate. |
A circus of materials to handle and discuss how they feel when touched.
An interesting thing to do is to let them pick up a small metal object and then enclose it in their hand - it soon becomes the temperature of the hand and feels 'warm' to another person... polystyrene feels cooler the longer you hold it within your fist. |
| Boiling is the maximum temperature a substance can reach. |
It is the maximum temperature of the liquid phase of the substance but not the gaseous one! |
|
| Boiling point of water is always 100oC |
They do not realise that the boiling point depends on air pressure and purity of the substance. |
Research into cooking on a mountain top - how eggs have to be 'boiled' for longer because the water boils at a lower temperature.
(This also involves understanding how heat is transferred through food by conduction/convection to cook it.) |
| When temperature at boiling remains constant, something is "wrong". |
|
Class practical of heating ice is an excellent activity - you can assess:
- how well they record and display data (tables and graphs),
- how good their general observational skills are and then
have a lesson discussing the results and observations - high level explanations like evaporation and condensation in cool air, dissolved gases in cool water escape when heated (the impact on fish!).Boiling bubbles - when and where they start etc.
Tip: use a graduated beaker and get them to note the volume of water and all ofther observations when they are doing this experiment. (They note down things like dissolved gas escaping at low temps (about 40 degrees) - 'steam' (water vapour escaping as the temperature increases (below boiling) and condenses in cool air or on the side of the beaker (as beaker gets hotter this condensation on beaker stops))...
|
| Steam can be 'seen' |
Steam is colourless |
We see the condensing steam above a kettle spout - but below that it is colourless (Care steam burns are dangerous!) |
| The bubbles in boiling water contain "air", "oxygen" or "nothing" |
They contain steam - water in its gaseous form - oxygen comes out at a much lower temperature |
Use 'blowing a bubble' to show that you would not get a bubble if you did not put something in it. |
| The temperature of ice is 0oC and cannot be lower |
|
Get ice from the lab techs at a very low temperature and show this warming up to zero - the lab tech will need to bring the ice in the lesson - not leave it on the bench for you! It will need to be in an insulated container with a tiny hole for your thermometer for this to work - ice gets 'hot' (to zero) pretty quickly - then stays at zero for a while because it has to give out the latent heat of fusion. Ice for a lab is usually around zero - very seldom the temperature it was when taken out of the freezer. Speed is vital! |
| Heat always rises |
Warm particles tend to rise within a fluid because of density difference within the fluid.... but in conduction direction does not matter... and heat radiation is given out equally in all directions |
A demo with a metal strip conducting heat energy down - wax can fix rivets onto the strip and they can see them drop off as the heat travels down the metal strip.
Discussion of convection currents - hot particles rise within the fluid, but later fall as they become less dense than the surroundings - but they have not lost all of the heat energy - they are still hot when they 'fall'
A radiant heater's rays can be directed down, across or up |
| Objects that readily become warm (good conductors of heat) do not readily become cold and vice versa. |
|
|






