Radioactivity: Multiple Choice Questions Q1. Which of the following best describes the decay constant for a radioisotope?
Q2. After 64 days the activity of a radioactive nuclide has fallen to one sixteenth of its original value. Calculate the half-life of the radioactive nuclide.
Q3. Radioactive decay is described as being spontaneous. In this context spontaneous means:
Q4. The ionising properties of radiations determine their penetration power.
Q5. Protactinium has a half life of 70s. A sample of protactinium is prepared and monitored over a period of time. Which of the following statements is correct?
Q6. Which of the following does not contribute to background radiation?
Q7. A radioactive source is placed 2.0 cm from a detector. The count rate decreases slightly if a sheet of paper is inserted between the source and the detector. It is reduced to background radiation level if the sheet of paper is replaced by a 1.0 cm thick sheet of aluminium. Deduce what forms of radiation the source emits:
Q8. Before carrying out a radioactivity experiment it is necessary to carry out a background radiation count (so that you can calculate the background count rate). The value of that count is not affected by:
Q9. Within a school laboratory you should always handle radioactive sources with long handled tongs and keep the time of use to a minimum. Choose from the choices below which form of radiation this safety advice most applies to, and for which reason.
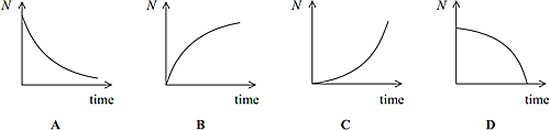
Q10. Some rocks contain lead as a product of radioactive decay. Via one such decay chain a fixed quantity of polonium decays to a stable isotope of lead. Which sketch graph best shows the number of lead atoms (N) in the sample as time progresses?
Q11. The sodium isotope Which line, A to D, in the table below correctly represents the production of
Q12. Artificial radioactive nuclides are manufactured by placing naturally-occurring nuclides in a nuclear reactor. They are made radioactive in the reactor as a consequence of bombardment by:
|
Follow me...
|