Solutions: Radioactivity Questions
Q5.
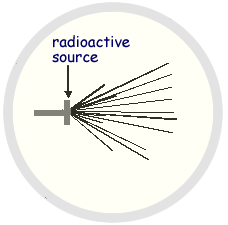 (a) The diagram is copied from a photograph taken of a cloud chamber containing a small radioactive source.
(a) The diagram is copied from a photograph taken of a cloud chamber containing a small radioactive source.
(i) What type of radiation is emitted from the source?
Alpha 
(ii) State and explain what can be deduced about the energy of the particles emitted by the source.
There are two different track lengths. Short range particles have lower energy than long range particles
Short range particles have lower energy than long range particles  The particles in each range have same energy.
The particles in each range have same energy. 
(4 marks)
(b) Plutonium–239 is a radioactive isotope that emits  particles of energy 5.1 MeV and decays to form a radioactive isotope of uranium. This isotope of uranium emits
particles of energy 5.1 MeV and decays to form a radioactive isotope of uranium. This isotope of uranium emits  particles of energy 4.5 MeV to form an isotope of thorium which is also radioactive.
particles of energy 4.5 MeV to form an isotope of thorium which is also radioactive.
(i) Write down an equation to represent the decay of plutonium–239.
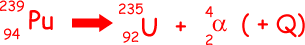

(ii) Write down an equation to represent the decay of the uranium isotope.


(iii) Which of the two radioactive isotopes, plutonium–239 or the uranium isotope, has the longer half-life? Give a reason for your answer.
U–235  because of there is an inverse relationship
between half–life and alpha particle energy
because of there is an inverse relationship
between half–life and alpha particle energy
(iv) Explain why thorium is likely to be a  emitter.
emitter.
It is likely to be a beta- emitter because the Th–90 nucleus is neutron–rich compared with U–235 [or Pu–239] 
(5 marks)
(Total 9 marks)


