Solutions: Radioactivity Questions
Q3.
(a) Nuclear fission can occur when a neutron is absorbed by a nucleus of uranium-235. An incomplete equation for a typical fission reaction is given below.
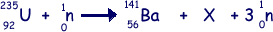
(i) State the nuclear composition of X.
proton number = 36 
neutron number = 56 
(ii) Name the element of which X is an isotope.
Krypton 
(3 marks)
(b) In a small nuclear power plant one fifth of the fission energy is converted into a useful output power of 10 MW. If the average energy released per fission is 3.2 × 10–11J, calculate the number of uranium-235 nuclei which will undergo fission per day.
It is only operating at one-fifth efficiency so
total output = 10 × 5 = 50 MW 
energy in one day = 50 × 106 × 24 × 3600 J  = 4.32 × 1012 J
= 4.32 × 1012 J
fission atoms per day = 4.32 × 1012 /3.2 × 10–11
= 1.35 ×1023
(3 marks)
(Total 6 marks)


