Solutions: Radioactivity Questions
Q17. The first artificially produced isotope, phosphorus-30, was formed by bombarding an aluminium-27 isotope,with an α particle.
(a) Complete the following nuclear equation by identifying the missing particle.
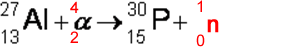

(1 mark)
(b) For the reaction to take place the α particle must come within a distance, d, from the centre of the aluminium nucleus.
Calculate 'd' if the nuclear reaction occurs when the α particle is given an initial kinetic energy of at least 2.18 × 10–12 J.
The electrostatic potential energy between two point charges Q1 and Q2 is equal to:
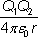
where
r is the separation of the charges and
ε0 is the permittivity of free space.
The kinetic energy lost by the α particle approaching the nucleus is equal to potential energy gain. 
Q1 = 2e (the charge on the alpha particle)
Q1 = 13e (the charge on the aluminium nucleus)
r = d
e = the magnitude of the charge on an electron = 1.6 x 10-19C
E = Q1Q2/4πε0r
2.18 × 10–12 = 13 x 2 (1.6 x 10-19)/4πε0d
d = 13 x 2 (1.6 x 10-19)/(4π x 8.85 x 10-12 x 2.18 × 10–12)
d = 2.75 x 10-15 m 
(3 marks)
(Total 4 marks)


