Solutions: Radioactivity Questions
Q10.
(a) Sketch, using the axes provided, a graph of neutron number, N, against proton number, Z, for stable nuclei over the range Z = 0 to Z = 80. Show suitable numerical values on the N axis.
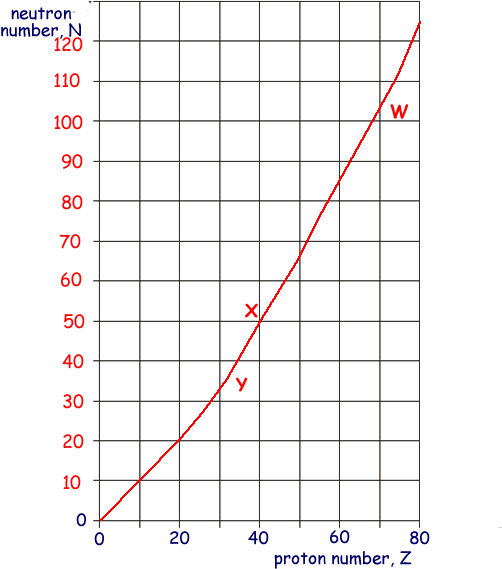
N increases as Z increases - Graph should pass through N = 10 when Z = 10 
N = (anywhere between 115 to 125) when Z = 80 
(2 marks)
(b) On the graph indicate, for each of the following, a possible position of a nuclide that may decay by
(i)  emission, labelling the position with W,
emission, labelling the position with W,
W should be placed at a value of Z that is greater than 60 just (within one diagonal of a square) below the line 
(ii)  emission, labelling the position with X,
emission, labelling the position with X,
X just (within one diagonal of a square) above line 
(iii)  emission, labelling the position with Y.
emission, labelling the position with Y.
Y just (within one diagonal of a square) below line 
(3 marks)
(c) The isotope  decays sequentially by emitting
decays sequentially by emitting  particles and
particles and  particles, eventually forming the isotope
particles, eventually forming the isotope  . Four
. Four  particles are emitted in the sequence. Calculate the number of
particles are emitted in the sequence. Calculate the number of  particles in the sequence.
particles in the sequence.
Only alphas change the nucleon number:
4 alphas will reduce the nucleon number by 16 (222 - 16 = 206)
Those 4 alphas will reduce the proton number by 2 x 4 = 8
80 - 8 = 78 
Betas will increase the proton number by 1.
The final proton number is 82 therefore there must be
82 - 78 = 4 beta particle emissions. 
(2 marks)
(d) A particular nuclide is described as proton-rich. Discuss two ways in which the nuclide may decay.
- β+ (positron) emission occurs for proton rich isotopes that are light (Z < 60) - a proton changes to a neutron reducing the proton number.
- Alpha particle emission occurs for proton rich heavy nuclei (Z > 60). An alpha particle emission reduces the mass of the nucleus by 4 and the number of protons by 2.
- Proton emission only occurs if the nuclide is very proton rich. This makes the nucleus highly unstable. Proton emission is a very rare process as the electrostatic repulsion has to overcome the strong nuclear force.
- Electron capture involves the nucleus capturing an electron which then combines with a proton, resulting in a proton changing into a neutron.
Marking: listing two processes discussing each of the two processes
discussing each of the two processes 

(3 marks)
(Total 10 marks)


