Solutions: Radioactivity Questions
Q1.
(a)
(i) Sketch a graph to show how the neutron number, N, varies with the proton number, Z, for naturally occurring stable nuclei over the range Z = 0 to Z = 90. Show values of N and Z on the axes of your graph and draw the N = Z line.
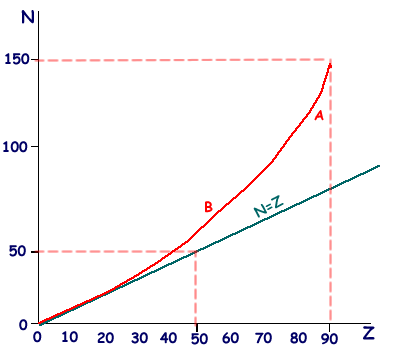
(ii) On your graph mark points, one for each, to indicate the position of an unstable nuclide which would be likely to be an  emitter, labelling it A, a
emitter, labelling it A, a  emitter, labelling it B.
emitter, labelling it B.
N=Z 
Scales on graph 
(5 marks)
(b) State the changes in N and Z which are produced in the emission of
(i) an  particle,
particle,
N decreases by 2; Z decreases by 2 
(ii) a  particle.
particle.
N decreases by 1; Z increases by 1 
(2 marks)
(c) The results of electron scattering experiments using different target elements show that

where A is the nucleon number and ro is a constant. Use this equation to show that the density of a nucleus is independent of its mass.
Denisty = mass/molume = m/V
Now, mass (m) is proportional to A
and volume (V) is proportional to R3, from the equation above we see that R is proportional to the cube root of A; therefore Volume V is proportional to A
Therefore density is proportional to A/A = 1.
i.e. density is independent of A.
(3 marks)
(Total 10 marks)


