Solutions for
Radioactivity: Carbon Dating
Q3.
The age of a piece of bone recovered from an archaeological site may be estimated by 14C dating.
All living organisms absorb 14C but there is no further intake after death. The proportion of 14C is constant in living organisms.
A 1 g sample of bone from an archaeological site has an average rate of decay of 5.2 Bq due to 14C.
A 1 g sample of bone from a modern skeleton has a rate of decay of 6.5 Bq. The counts are corrected for background radiation.
Calculate the age, in years, of the archaeological samples of bone.
Half-life of 14C = 5730 years
(4 marks)
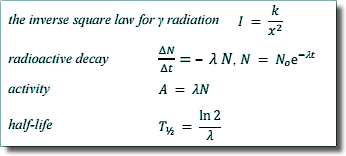
λ = ln 2/Thalf
λ = ln 2/5730
λ = 1.21 x 10-4 y-1 
Nt = Noe-λ
t
so, At = Aoe-λ
t
5.2/6.5 = e-λ
t
Taking logs: ln (5.2/6.5) = - λ
t 
t = 0.223/1.21 x 10-4
t = 1840 years







