Particle Physics Questions : Multiple Choice
Q1. What are the numbers of hadrons, baryons and mesons in an atom of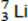 ?
?
|
hadrons |
baryons |
mesons |
A |
7 |
3 |
3 |
B |
7 |
4 |
4 |
C |
7 |
7 |
0 |
D |
10 |
7 |
0 |
Q2. A radioactive nucleus emits a β– particle then an α-particle and finally another β– particle.
The final nuclide is:
A |
an isotope of the original element |
B |
the same element with a different proton number |
C |
a new element of higher proton number |
D |
a new element of lower nucleon number |
Q3. The nucleus of  captures a proton and emits an α particle.
captures a proton and emits an α particle.
What is the product nucleus?
A 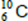
B 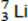
C 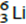
D 
Q4. Electron capture can be represented by the equation:
p + e- → X + Y
Which row correctly identifies X and Y?
|
X |
Y |
A |
p |
K- |
B |
e- |
e+ |
C |
n |
νe |
D |
n |
π0 |
Q5. A calcium ion is formed by removing two electrons from an atom of 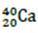 .
.
What is the specific charge of the calcium ion?
A |
3.2 × 10-19 C kg-1 |
B |
2.9 × 10-18 C kg-1 |
C |
4.8 × 106 C kg-1 |
D |
4.8 × 107 C kg-1 |
Specific charge is charge per kilogram (it tells you that by giving you the unit!)
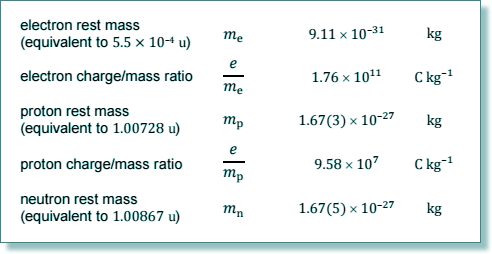
charge/mass of ion = (2e/(20mp + 20mn)
charge/mass of ion = 2e/40mp
charge/mass of ion = 2 x 1.6 x 10-19/(40 x 1.67 x 10-27)
charge/mass of ion = 4.8 × 106 C kg-1
Q6. The table below links exchange particles with nuclear processes. Select the line that does not give the correct exchange particle for the process.
| |
Process |
Exchange Particle |
| A |
gravitational attraction |
Z boson |
| B |
electrostatic repulsion of electrons |
virtual photon |
| C |
strong interaction |
pion |
| D |
β− decay |
W boson |
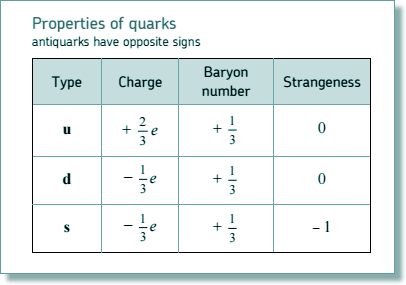
Q7. Select the line that gives the correct category and quark combination for the particle.
| |
Particle |
Category |
Quark combination |
| A |
neutron |
baryon |
̅u̅ d |
| B |
neutron |
meson |
u d d |
| C |
proton |
baryon |
u u d |
| D |
positive pion |
meson |
̅u̅ d |
Q8. Select the incorrect statement about muons from the following.
| A |
A muon is a lepton |
| B |
A muon has a greater mass than an electron. |
| C |
If a muon and an electron each have the same de Broglie wavelength then they each have the same momentum. |
| D |
A muon with the same momentum as an electron has a larger kinetic energy than the electron. |
Q9. Which of the following nuclei has the smallest specific charge?
Q10.  is an unstable nuclide in a radioactive decay series. It decays by emitting an α particle. The next two nuclides in the series emit β− particles. Determine which nuclide is formed after these three decay processes have taken place.
is an unstable nuclide in a radioactive decay series. It decays by emitting an α particle. The next two nuclides in the series emit β− particles. Determine which nuclide is formed after these three decay processes have taken place.
Q11.
A CERN detector shows a signal only in the hadronic calorimeter. No signal is observed in the in the tracker, electromagnetic calorimeter or muon chambers). Therefore, this signal is most likely due to a:
| A |
pion |
| B |
neutrino |
| C |
photon |
| D |
neutron |
Q12.
The exchange particles carrying the strong nuclear force are the:
| A |
photons |
| B |
gluons |
| C |
gravitons |
| D |
w-bosons |
Q13.
Which was the first particle discovered which is still today believed to be elementary, i.e. not made up of further constituents?
| A |
electron |
| B |
gluon |
| C |
photon |
| D |
proton |
Q14. Which of the following is not true?
A |
Each meson consists of a single quark and a single antiquark. |
B |
Each baryon consists of three quarks. |
C |
The magnitude of the charge on every quark is 1/3 |
D |
A particle consisting of a single quark has not been observed. |