Particle Physics Questions
Q1.
(a) Describe how the strong nuclear force between two nucleons varies with the separation of the nucleons quoting suitable values for separation. (3 marks)
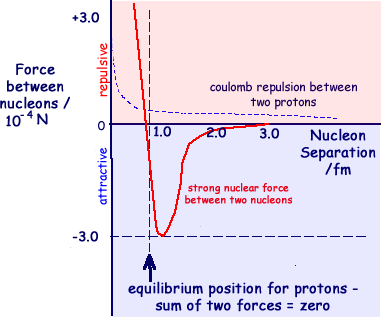
At a spearation of less than 0.5 fm the force is repulsive. It then becomes attractive over a short range,
It then becomes attractive over a short range,  peaking at 1.3 fm at a force of 10-4 N. and becoming negligible by about 3 fm separation.
peaking at 1.3 fm at a force of 10-4 N. and becoming negligible by about 3 fm separation.
(b) An unstable nucleus can decay by the emission of an alpha particle.
(i) State the nature of an alpha particle. (1 mark)
helium nucleus OR 2 protons and 2 neutrons 
(ii) Complete the equation below to represent the emission of an alpha particle by a uranium nucleus.
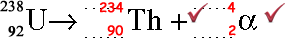
(2 marks)
(c) Uranium 238 decays in stages by emitting  particles and
particles and  particles, eventually forming
particles, eventually forming  a stable isotope of lead, called lead 206.
a stable isotope of lead, called lead 206.
(i) State what is meant by 'isotope'. (2 marks)
Isotopes of an element have the same number of protons  as each other, but a different number of neutrons.
as each other, but a different number of neutrons. (This results in the same chemical properties but different physical properties).
(This results in the same chemical properties but different physical properties).
(ii) If there are eight alpha decays involved in the sequence of decays from uranium 238 to lead 206 deduce how many  decays are involved. (show your working!)
decays are involved. (show your working!)
Proton number change: 92 - 82 = 10
8 alpha decays means proton number decreases by 16.
It only deceases by 10 so there must be 6 beta decays.
(3 marks)
(Total 11 marks)


