A Level Specific Heat Capacity Questions
Q4.
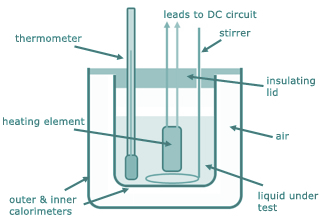
(a) A student immerses a 2.0kW electric heater in an insulated beaker of water. The heater is switched on and after 120 s the water reaches boiling point. The data collected during the experiment is given below.
initial mass of beaker |
25 g |
initial mass of beaker and water |
750 g |
initial temperature of water |
20 °C |
final temperature of water |
100 °C |
Calculate the specific heat capacity of water if the thermal capacity of the beaker is negligible.
(4 marks)
(b) The student in part (a) continues to heat the water so that it boils for 105 s. When the mass ofthe beaker and water is measured again, it is found that it has decreased by 94 g.
(i) Calculate a value for the specific latent heat of vaporisation of water.
(ii) State two assumptions made in your calculation.
(4 marks)
[Total 8 marks]



