A Level Specific Heat Capacity Questions
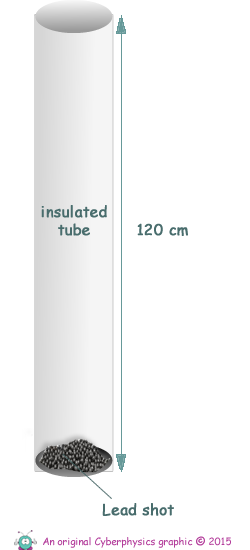 Q9. The diagram on the right shows a tube containing small particles of lead, called 'lead shot'. When the tube is inverted the particles of lead fall freely through a vertical height equal to the length of the tube.
Q9. The diagram on the right shows a tube containing small particles of lead, called 'lead shot'. When the tube is inverted the particles of lead fall freely through a vertical height equal to the length of the tube.
(a) Describe the energy changes that take place in the lead particles during one inversion of the tube.
Lead particles fall and lose potential energy (as tube is inverted).  This is converted to kinetic energy.
This is converted to kinetic energy.  Kinetic energy is converted into heat energy on impact.
Kinetic energy is converted into heat energy on impact. 
(3 marks)
(b) The tube is made from an insulating material and is used in an experiment to determine the specific heat capacity of lead.
The following results are obtained:
| mass of lead: |
0.025 kg |
| number of inversions: |
50 |
| length of tube: |
1.2m |
| change in temperature of the lead: |
4.5K |
Calculate
(i) the change in potential energy of the lead as it falls after one inversion down the tube,
ΔEp = mgΔh = 0.025 × 9.81 × 1.2 = 0.29(4) J 
(ii) the total change in potential energy after 50 inversions,
total change of energy = (50 × 0.294) = 15 J 
(iii) the specific heat capacity of the lead.
ΔQ = mcΔθ 14.7 = 0.025 × c × 4.5 
c = 131 J kg−1 K−1 
(4 marks)
(Total 7 marks)


