Nuclear Radius
Q3. Nuclear radii can be determined by observing the diffraction of high energy electrons, as shown in the diagram.
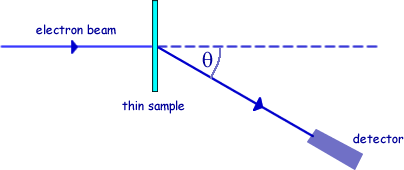
(a) On the axes below, sketch a graph of the results expected from such an electron diffraction experiment.
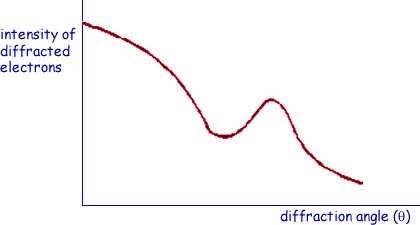
graph shows a minimum  which does not touch the axis
which does not touch the axis 
(2 marks)
(b) State why high energy electrons are used in determining nuclear size.
The de Broglie wavelength of high energy electrons is comparable to nuclear radii, they can therefre be diffracted by nuclei [or not subject to the strong nuclear force] 
(1 mark)
(c) Electron diffraction experiments have been performed on a range of different nuclei to give information about nuclear density and average separation of particles in the nucleus. Give the main conclusion in each case.
Nuclear density has been found to be constant.  The separation of neighbouring nucleons has been found to be equal - regardless of whether they ar protons or neutrons [or nucleons are close-packed]
The separation of neighbouring nucleons has been found to be equal - regardless of whether they ar protons or neutrons [or nucleons are close-packed] 
(2 marks)
(d) Sketch a graph of the relationship between the radius of a nucleus and its nucleon number.
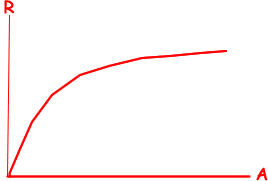

(1 mark)
(e) Given that the radius of the 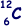 nucleus is 3.04 × 10–15m, calculate the radius of the
nucleus is 3.04 × 10–15m, calculate the radius of the 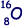 nucleus.
nucleus.
R = r0A1/3
So, RO/AO1/3 = RC/AC1/3
RO= RC AO1/3/AC1/3 = RC {AO/AC}1/3
R0 = 3.04 × 10–15 × (16/12)1/3
R0 = 3.35 × 10–15 m 
(3 marks)
(Total 9 marks)


