Rutherford Scattering - A Level Standard Questions
Q4. The diagram below shows the apparatus used to investigate Rutherford scattering, in which alpha particles are fired at a gold foil.
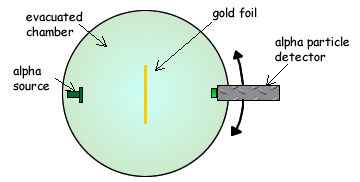
(a) Why is it essential for there to be a vacuum in the chamber?
There must be a vacuum in the chamber to prevent the alpha particles being absorbed or scattered  by air molecules
by air molecules 
(2 marks)
(b) What observations made with this apparatus support each of the following conclusions? No explanation is required.
(i) The nuclear radius of gold is much smaller than its atomic radius.
There is little or no deflection  by a majority of alpha particles
by a majority of alpha particles 
(ii) Most of the mass of an atom of gold is contained in its nucleus.
Some alpha particles suffer large deflection [or backscattering occurs] 
(3 marks)
(c) The drawing below shows alpha particles incident on a layer of atoms in a gold foil. Copy this figure draw the complete path followed by each of the alpha particles shown.
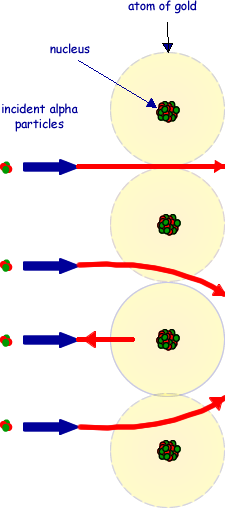
First path continues undeflected 
Second path deflected downwards and fourth path deflected upwards 
Third path shows backscattering (inside the dotted circle) 
(3 marks)
(Total 8 marks)


