Quantum Phenomena - discrete energy levels for electrons
Q5. The diagram below shows the lowest three energy levels of a hydrogen atom.
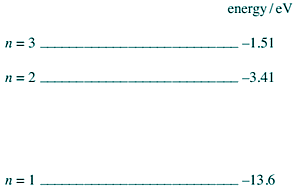
(a) An electron is incident on a hydrogen atom. As a result an electron in the ground state of the hydrogen atom is excited to the n = 2 energy level. The atom then emits a photon of a characteristic frequency.
(i) Explain why the electron in the ground state becomes excited to the n = 2 energy level.
(2 marks)
(ii) Calculate the frequency of the photon.
(3 marks)
(iii) The initial kinetic energy of the incident electron is 1.70 × 10−18 J. Calculate its kinetic energy after the collision.
(2 marks)
(iv) Show that the incident electron cannot excite the electron in the ground state to the n = 3 energy level.
(2 marks)
(b) When electrons in the ground state of hydrogen atoms are excited to the n = 3 energy level, photons of more than one frequency are subsequently released.
(i) Explain why different frequencies are possible.
(1 mark)
(ii) State and explain how many possible frequencies could be produced.
(2 marks)
(Total 12 marks)








