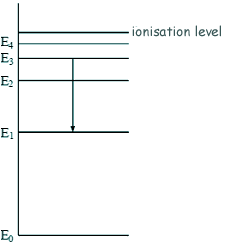
Energy Transistions - Multiple Choice Q1.The diagram shows some energy levels of an atom.
The transition E3 to E1 corresponds to the emission of visible light. A transition corresponding to the emission of infrared radiation could be:
All transitions to E1 will be visible.... so we are looking for a smaller transition - a transition to E2 (choice C or D) The photon is emitted not absorbed so the transition is to a lower energy number (choice D).
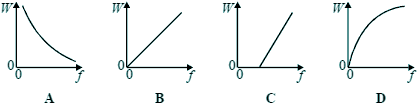
Q2. Which one of the graphs below best represents the relationship between the energy W of a photon and the frequency f of the radiation?
E=hf or in this case W = hf the equation of a straightline with c=0 (no intercept) Choice B Q3. The diagram shows some of the energy levels for a hydrogen atom.
A free electron of kinetic energy 20.0 × 10–19 J collides with a hydrogen atom in its ground state. The hydrogen atom is excited from its ground state to the first excited state. Calculate the kinetic energy of the free electron after the collision in joules.
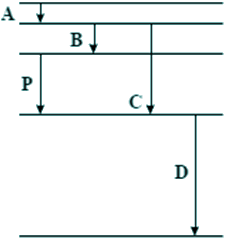
The electron absorbs the whole photon's energy. It uses (21.8 - 5.4) × 10–19 J of it to make the jump - 16.4 × 10–19 J. That leaves (20.0 - 16.4) × 10–19= 3.6 × 10–19J - choice B Q4. The diagram below is drawn to scale. It shows some of the energy levels of an atom.
Transition P results in the emission of a photon of wavelength 4 × 10–7 m. Which one of the transitions A, B, C, or D could result in the emission of a photon of wavelength 8 × 10–7 m? Wavelength is inversely proportional to frequency and therefore energy. Therefore the doubling of wavelength means halving the energy. Line B is half the length of line P - Choice B is the answer. |
Follow me...
|