A level: Kinetic Theory Questions
Q9.
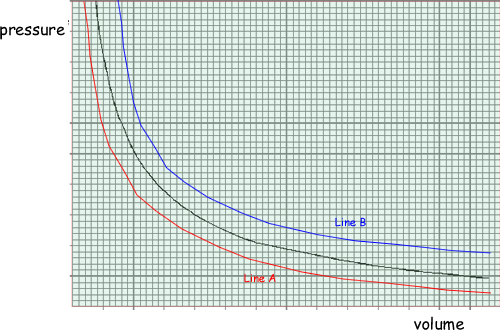
The graph shows how the pressure of an ideal gas varies with its volume when the mass and temperature of the gas are constant.
(a) On the same axes, sketch two additional curves A and B, if the following changes are made.
(i) The same mass of gas at a lower constant temperature (label this A).
(ii) A greater mass of gas at the original constant temperature (label this B).
See graph: curve A below original, curve B above original  both curves correct shape
both curves correct shape 
(2 marks)
(b) A cylinder of volume 0.20 m3 contains an ideal gas at a pressure of 130 kPa and a temperature of 290 K. Calculate
(i) the amount of gas, in moles, in the cylinder,

n = pV/RT
= 130 x 103 x 0.20/8.31 x 290 
= 10.4
= 11 mol
(ii) the average kinetic energy of a molecule of gas in the cylinder,
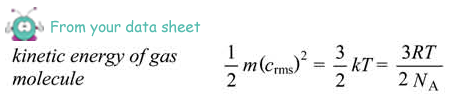
Ek = 3/2 × 1.38 × 10–23 × 290 
= 6.0 × 10–21 J 
(iii) the average kinetic energy of the molecules in the cylinder.
Total KE = KE of one average molecule x number of molecules
= KE of one average molecule x number of moles x Avogadro Number
= 6.0 × 10–21 x 10.8 × 10.8 x 6.02 × 1023
= 3.9 × 104 J 
(5 marks)
(Total 7 marks)


