A level: Kinetic Theory Questions
Q5.
(a) The volume of air in a fully expanded pair of human lungs is 5.0 × 10–3 m3. The pressure of the air in the lungs is 1.0 × 105 Pa and its temperature is 310 K.
Calculate:
(i) the number of moles of air in the lungs,

pV = nRT
n = pV/RT
= 1.0 × 105 x 5.0 × 10–3/(8.31 x 310) 
= 0.194 moles 
(ii) the average kinetic energy of an air molecule in the lungs.
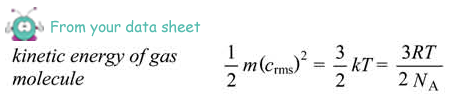
average kinetic energy = 3/2 × 1.38 × 10-23 × 310 
= 6.4 × 10–21J 
(4 marks)
(b) Air is a mixture of oxygen and nitrogen molecules. The mass of an oxygen molecule is greater than the mass of a nitrogen molecule. State and explain the effect this has on the mean square speeds of the oxygen and nitrogen molecules in the lungs.
The molecules are at the same temperature therefore they will have the same average kinetic energy.  Kinetic energy is 1/2mv2
Kinetic energy is 1/2mv2  therefore the nitrogen molecules must have a higher mean square speed
therefore the nitrogen molecules must have a higher mean square speed  as they have a smaller mass.
as they have a smaller mass.
(3 marks)
(Total 7 marks)


