A level: Kinetic Theory Questions
Q15. The diagram shows an arrangement that is used to measure the density of a powder.
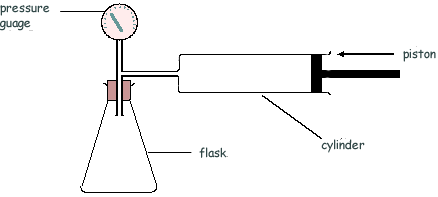
The air in the cylinder is forced into the flask and the air pressure is measured, by the pressure gauge P, before and after the change. The test is then repeated with the powder present in the flask.
In both tests, the initial pressure in the flask is the same.
(a)
(i) Explain why, after compression of the air the pressure in the flask is greater when the powder is present than when it is not present.
The volume of air is less with the powder present as the poweder takes up some of the room in the flask.  As pressure is inversely proportional to volume (at constant temperature) the decrease in volume causes an increase in pressure.
As pressure is inversely proportional to volume (at constant temperature) the decrease in volume causes an increase in pressure.
(ii) Calculate the pressure in the flask, after compression at constant temperature, when no powder was present. Assume that the volume of the tubes and pressure gauge is negligible.
 volume of the empty flask = 2.50 × 10–4 m3
volume of the empty flask = 2.50 × 10–4 m3
 volume of the cylinder = 1.00 × 10–4 m3
volume of the cylinder = 1.00 × 10–4 m3
 initial pressure of the air in the flask and cylinder = 100 kPa.
initial pressure of the air in the flask and cylinder = 100 kPa.
initial volume V1 = 3.5 × 10–4 m3
final volume V2 = 2.5 × 10–4 m3 
p1V1 = p2V2
p2 = p1V1 / V2
p2 = 100 x 103 x 3.5 × 10–4/(2.5 × 10–4) 
p2 = 140 x 103 Pa 
(6 marks)
(b) To test the apparatus, 0.13 kg of powder of density 2700 kg m–3 was placed in the flask before compression.
(i) Calculate the volume of this amount of powder.
density = mass/volume
volume = mass/density = 0.13/2700 = 4.8 × 10–5 m3
(ii) The pressure of the air in the flask increased to 150 kPa when the test was carried out with this amount of powder in the flask. By carrying out an appropriate calculation, justify whether or not the test was successful
initial volume = (3.5 – 0.48) × 10–4 m3 
final volume = (2.5 – 0.48) × 10–4 m3 
p1V1 = p2V2
p2 = p1V1 / V2
p2 = 100 x 103 x 3.02 × 10–4/(2.02 × 10–4)
p2 = 150 x 103 Pa 
The test was successful as the calculated final pressure was equal to the measured final pressure 
(5 marks)
(Total 11 marks)







