A level: Kinetic Theory Questions
Q11.
(a)
(i) One of the assumptions of the kinetic theory of gases is that molecules make elastic collisions. State what is meant by an elastic collision.
An elastic collision is a collision in which kinetic energy is conserved
(ii) State two more assumptions that are made in the kinetic theory of gases.
Any two of the following:
- molecules of a gas are identical [or all molecules have the same mass]

- molecules exert no forces on each other except during impact

- motion of molecules is random [or molecules move in random directions]

- volume of molecules is negligible (compared to volume of container) [or very small compared to volume of container or point particles]

- time of collision is negligible (compared to time between collisions)

- Newton's laws apply

- large number of particles (so the mathematics of statistics can be applied)

(3 marks)
(b) One mole of hydrogen at a temperature of 420 K is mixed with one mole of oxygen at 320 K. After a short period of time the mixture is in thermal equilibrium.
(i) Explain what happens as the two gases approach and then reach thermal equilibrium.
The hot gas cools and cooler gas heats up  until they are at same temperature.
until they are at same temperature.
OR Hydrogen molecules transfer energy to oxygen molecules  until average kinetic energy of both gases is the same.
until average kinetic energy of both gases is the same. 
(2 marks MAX)
(ii) Calculate the average kinetic energy of the hydrogen molecules before they are mixed with the oxygen molecules.
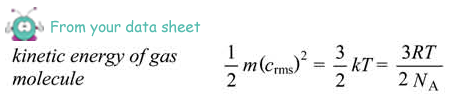
Ek = 3/2 × 1.38 × 10–23 × 420 
= 8.7 × 10–21 J 
(4 marks)
(Total 7 marks)


