GCSE Questions: Radioactivity
Q9. The diagram below shows the paths of two alpha particles A and B into and out of a thin piece of metal foil.
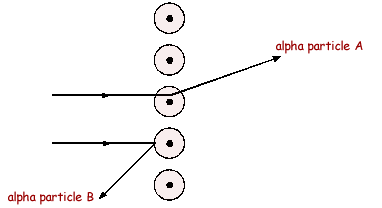
(a) The paths of the alpha particles depend on the forces on them in the metal. Describe the model of the atom which is used to explain the paths of alpha particles aimed at thin sheets of metal foil.
The model describes the atom as having:
A nucleus  with positive charge / protons in nucleus
with positive charge / protons in nucleus  and electrons / negative charges orbiting nucleus
and electrons / negative charges orbiting nucleus 
(3 marks)
(b) Scientists used to believe that atoms were made up of negative charges embedded in a positive 'dough'. This is called the 'plum pudding' model of the atom.
The diagram below shows a model of such an atom.
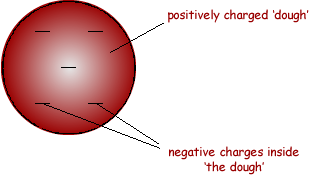
(i) Explain how the 'plum pudding' model of the atom can explain why alpha particle A is deflected through a very small angle.
The positive 'dough' repels the positive alpha particles (or two positive charges repel) 
but the charge is spread out over a relatively large volume so the repulsion forces would be small 
(2 marks)
(ii) Explain why the 'plum pudding' model of the atom can not explain the large deflection of alpha particle B.
A large force would be needed to backscatter the alpha particle,  the positive charge in the plum pudding is too spread out to do that
the positive charge in the plum pudding is too spread out to do that so the positive charge must be concentrated in a nucleus to explain the backscattering.
so the positive charge must be concentrated in a nucleus to explain the backscattering. 
(3 marks)
(c) We now believe that atoms are made up of three types of particles called protons, neutrons and electrons.
Complete the table below to show the relative mass and charge of a neutron and an electron.
The relative mass and charge of a proton have already been done for you.
PARTICLE |
RELATIVE MASS |
RELATIVE CHARGE |
proton |
1 |
+1 |
neutron |
1 |
0 |
electron |
|
-1 |

 - one mark fro each correct row.
- one mark fro each correct row.
(2 marks)
(d) The diagrams below show the nuclei of four different atoms A, B, C and D.
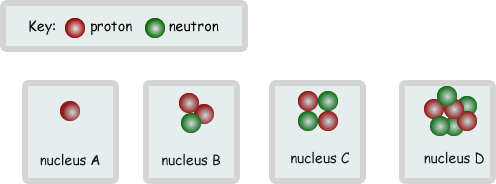
(i) State the mass number of C.
4 
(ii) Which two are isotopes of the same element? Explain your answer.
B and C  - they have the same number of protons as each other,
- they have the same number of protons as each other, but different numbers of neutrons.
but different numbers of neutrons.
(4 marks)
(Total 14 marks)









