Energy Transfer Questions - GCSE Level
Q3. A student was asked to investigate the heat loss from two metal cans, L and M. The cans were identical except for the outside colour.
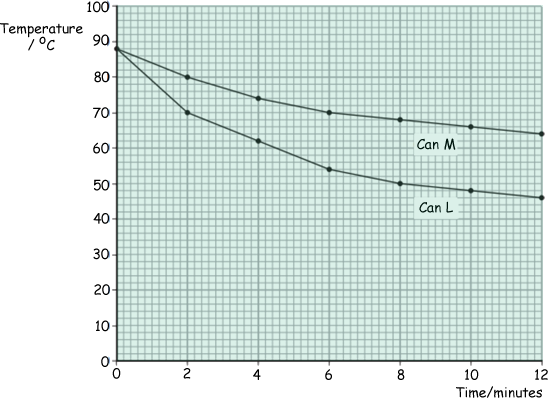
The student filled the two cans with equal volumes of hot water. He then placed the temperature sensors in the water and started the data logger. The computer used the data to draw the graph below.
(a) Which one of the following is a categoric variable?
A) the outside colour of the cans 
B) the starting temperature of the hot water
C) the time the volume of hot water
(1 mark)
(b) For can L, state the temperature drop of the water:
(i) in the first two-minute interval (1 mark)
18 °C or it dropped from 88°C to 70°C 
(ii) in the second two-minute interval (1 mark)
8 °C or it dropped from 70°C to 62°C 
(c) In both cans the water cooled faster at the start of the investigation than at the end of the investigation. Why? (1 mark)
Because at the start there was a greater temperature difference between the water and the surroundings. 
(d) One can was black on the outside and the other can was white on the outside. What colour was can L? Explain the reason for your answer. (3 marks)
Can L was black.
Temperature falls the fastest  in can L, this is because black is (a good/the best/a better) emitter (of heat/radiation).
in can L, this is because black is (a good/the best/a better) emitter (of heat/radiation). 
(7 marks total)








