Academic Applications>
Industrial applications
Medical Applications
Other Applications
|
|
Alpha
|
Beta
|
Gamma
|
Positron
|
|
Short
half life
 Tracers
in industry - detecting leaks in pipes Tracers
in industry - detecting leaks in pipes
 Tracers in botony experiments - e.g. phosphorus 32 is a beta emitter - taken up by the plant - can be detected outside the plant as beta penetrates thin plant structures easily - half life of 14 days makes it ideal for this. Tracers in botony experiments - e.g. phosphorus 32 is a beta emitter - taken up by the plant - can be detected outside the plant as beta penetrates thin plant structures easily - half life of 14 days makes it ideal for this.
|
Short
half life
 Medical
tracer - used with gamma camera Medical
tracer - used with gamma camera
 Tracers
in industry - detecting routes of underground rivers and streams Tracers
in industry - detecting routes of underground rivers and streams
|
Short
half life
 Medical
tracer - PET Scanning Medical
tracer - PET Scanning
|
|
Long
half life
 Dating
of rocks using Uranium-238/lead ratios Dating
of rocks using Uranium-238/lead ratios
 Smoke
detectors Smoke
detectors
 Gas
lamp mantles Gas
lamp mantles
 Nuclear
batteries Nuclear
batteries
|
Long
half life
 Thickness
control of very thin metal sheets, paper or cardboard in manufacturing
and industry Thickness
control of very thin metal sheets, paper or cardboard in manufacturing
and industry
 C-14
dating C-14
dating
 Emergency
sign lighting Emergency
sign lighting
|
Long
half life
 High
activity - radiotherapy High
activity - radiotherapy
 High
activity - sterilisation of medical surgical instruments High
activity - sterilisation of medical surgical instruments
 High
activity - irradiation of food to kill bacteria and prolong
shelf life High
activity - irradiation of food to kill bacteria and prolong
shelf life
 Thickness
control of metal sheets (when too thick for beta) in manufacturing
and industry Thickness
control of metal sheets (when too thick for beta) in manufacturing
and industry
 Checking
welds Checking
welds
|
|
The most common and accepted
method of 'absolute geologic dating' (establishment of actual age) is
based on the natural radioactivity
of certain minerals found in rocks. As the rate
of radioactive decay of any particular isotope
is known, the age of a specimen can be worked out from the ratio of the
remaining isotope and its decay product.
 Dating of Igneous
Rocks (Using Uranium Content)
Dating of Igneous
Rocks (Using Uranium Content)
Geologists use this
method to date igneous rock samples. If you look carefully at the half-lives
of isotopes in the Uranium series you appreciate that the Uranium has
a much longer half-life than any of the others. See the Uranium decay
series(4N+2).
So, by comparing the
proportion of Uranium in the rock to the proportion of Lead produced by
its decay you can work out how many half-lives it has been decaying.
Then by using the
half-life of Uranium you can work out the time involved.
|
Percentage
of Uranium
|
Percentage
of Lead
|
Ratio
of Uranium to Lead
|
Age
(millions of years)
|
| Initial
Value |
100.00%
|
0.00%
|
1:0
|
0
|
| After
one half-life |
50.00%
|
50.00%
|
1:1
|
4,500
|
| After
two half-lives |
25.00%
|
75.00%
|
1:3
|
9,000
|
| After
three half-lives |
12.50%
|
87.50%
|
1:7
|
13,500
|
| After
four half-lives |
6.25%
|
93.75%
|
1:15
|
18,000
|
 Dating
of Ancient Artefacts (Carbon Dating)
Dating
of Ancient Artefacts (Carbon Dating)
Carbon
dating measures the
remaining amount of the radioactive isotope carbon-14 in organic matter.
It can be used to date specimens as old as 35,000 years.
During its lifetime
a biological entity (plant or animal) takes an active part in the carbon
cycle and it contains the same proportion of the isotope as the atmosphere
does (about one ten millionth of the carbon is carbon-14).
The death of an organism
terminates the incorporation of this isotope into the fabric of the entity.
From the time of death onwards the proportion of carbon-14 decreases as
it decays into nitrogen.
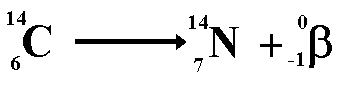
By calculating the
ratio of C-14 to total carbon in a sample of the artefact it is possible
to work out its age. The half-life of carbon-14 is 5,600 years.
E.g.
|
C-14
in total carbon |
Age
(years)
|
| Initial
Value |
1
part in 10 million |
0
|
| After
one half-life |
0.5
parts in 10 million |
5,600
|
| After
two half-lives |
0.25
parts in 10 million |
11,200
|
| After
three half-lives |
0.125
parts in 10 million |
16,800
|
| After
four half-lives |
0.0625
parts in 10 million |
22,400
|

 Tracers in Industry
Tracers in Industry
 Leaks from a pipeline
can be traced by adding a radioactive isotope into what ever it is carrying.
The source must have a short half-life (a few hours) so that it can
be detected as it passes through but not stay radioactive long enough
to pose a health hazard.
Leaks from a pipeline
can be traced by adding a radioactive isotope into what ever it is carrying.
The source must have a short half-life (a few hours) so that it can
be detected as it passes through but not stay radioactive long enough
to pose a health hazard.
 Wear of moving
parts can be tested by making the part radioactive and monitoring the
proportion of worn parts in the lubricating oil by looking for the level
of radioactivity in it. See this page.
Wear of moving
parts can be tested by making the part radioactive and monitoring the
proportion of worn parts in the lubricating oil by looking for the level
of radioactivity in it. See this page.
 A gamma source
can be used to check welds in metal parts. It is used in a similar way
to X-rays on a human body. A photographic plate is placed behind the
weld. It is exposed more where the weld is weak.
A gamma source
can be used to check welds in metal parts. It is used in a similar way
to X-rays on a human body. A photographic plate is placed behind the
weld. It is exposed more where the weld is weak.
 Sterilisation of Food and Surgical Instruments Sterilisation of Food and Surgical Instruments
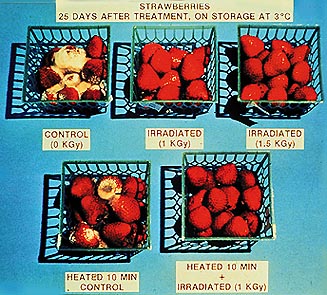 Gamma rays kill bacteria. Therefore irradiating food or surgical instruments is a good way of ensuring they are sterile. The gamma rays penetrate packaging, so the food or instrument can be sealed and then sterilised so that re-contamination cannot occur. Gamma rays kill bacteria. Therefore irradiating food or surgical instruments is a good way of ensuring they are sterile. The gamma rays penetrate packaging, so the food or instrument can be sealed and then sterilised so that re-contamination cannot occur.
No radioactive source particles are allowed to get in touch with the irradiated substance. The source is sealed so that only gamma rays get out. Therefore the irradiated substance is sterile but NOT radioactive. |
 Thickness Control in Manufacturing Thickness Control in Manufacturing
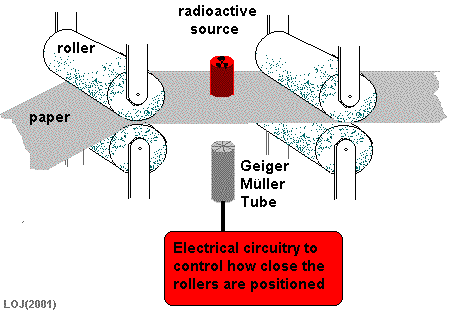 Automatic control over the thickness of paper in paper mills can be obtained by passing beta radiation through the paper and monitoring the count rate. An isotope with a long half-life is used so that the count-rate hardly changes with time. Electrical circuitry is then set up to ensure that a constant rate is maintained. If the rate is too low the rollers automatically move closer to each other (making the paper thinner) and vice versa. Automatic control over the thickness of paper in paper mills can be obtained by passing beta radiation through the paper and monitoring the count rate. An isotope with a long half-life is used so that the count-rate hardly changes with time. Electrical circuitry is then set up to ensure that a constant rate is maintained. If the rate is too low the rollers automatically move closer to each other (making the paper thinner) and vice versa.
|
 Nuclear Batteries Nuclear Batteries
 The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions. The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions.
|

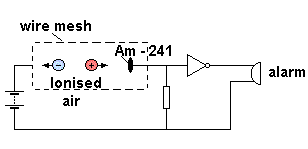 Smoke Detectors
Smoke Detectors
Some smoke detectors contain a small amount of Americium-241, an alpha emitter (and low energy gamma emitter) with a half life of 460 years. It consists of an ionisation chamber linked to a simple electronic alarm circuit The Americium ionises the air between the plates, causing a current to flow. Smoke entering the detector blocks some of the alpha particles, lowering the current, and triggering the alarm.
 Gas Lamp Mantles Gas Lamp Mantles
 Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see decay series). It apparently improved the flame. This practice has been stopped but old stock may still be around. Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see decay series). It apparently improved the flame. This practice has been stopped but old stock may still be around.
|
 Emergency Exit Sign Lighting Emergency Exit Sign Lighting
 During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years. During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years. |
 'Glow In The Dark'
Watches
'Glow In The Dark'
Watches
All radium dial watches should be disposed of properly. Above is a demonstration of the radioactivity from a radium-containing 1950's Timex watch dial, using Geiger counter.
They also use tritium,
see emergency exit signs above.
 Vaseline Glass Vaseline Glass
 Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-green. It glows under ultraviolet rays. Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-green. It glows under ultraviolet rays.
|
 'Glow In The Dark' Clock Hands 'Glow In The Dark' Clock Hands
 Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document: Radium Dial Painting and Its Tragic Consequences Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document: Radium Dial Painting and Its Tragic Consequences
|
Medical
Applications
Nuclear radiation is
used in two ways in medicine:
- as
a tracer
(also see PET scans) - Radioactive
isotopes and radioactively labeled molecules are used
as tracers to identify abnormal bodily processes. This
is possible because some natural elements tend to concentrate
in certain parts of the body: iodine in the thyroid, phosphorus
in the bones, potassium in the muscles. When a patient
is injected with a radioactive element, a special camera
can take pictures of the internal workings of the organ.
- as
a medical treatment for cancer (radiotherapy)
|
| |
Radioactive
Tracer
|
Radioactive
Treatment
|
| Type
of treatment |
Diagnostic |
Therapy |
| Aim
of treatment |
To
investigate the function of a part of the body by labelling
a biologically useful compound with radioactive atoms |
To
destroy malignant tumours with a high dose of radiation that will
result in cell
death |
| Type
of dose administered |
Minimal
dose
to patient |
Maximum
dose
to affected part, minimum dose to surrounding tissue |
| Type
of radiation used |
Gamma
Rays |
Gamma
Rays |
| Example
of substances used |
Pure
gamma emitters such as technetium
99m |
Pure
gamma emitters such as cobalt
60 and caesium 137 |
| Half
life |
Short
( about 6 hours) |
Long
(typically 5.3 years) |
| Treatment |
Radioactive
substance is injected into the patient making him/her mildly radioactive.
The nuclear radiation emitted is then 'viewed' using a gamma
camera |
A
strong radioactive source is used to deliver nuclear
radiation to the affected part. If this is from outside the
body the patient doesn't become radioactive BUT if it is from an
implanted source (like a radioactive wire inserted into the tumour)
the patient does become radioactive and usually has to stay in hospital
until the source is removed. |
| What
equipment is used? |
The
'hardware' in the hospital (a gamma
camera) does not deliver radiation but detects it. |
The
hardware in the hospital (a LINAC
- linear accelerator or Cobalt 60 unit) produces ionising
radiation which is 'fired' at the patient. |
| How
does the patient feel afterwards? |
After
the investigation the patient does not feel unwell |
After
the treatment the patient may well feel unwell: sickness, nausea,
exhaustion. |
| Is
the patient radioactive
afterwards? |
After
the investigation the patient is still mildly radioactive
and may need to avoid contact with pregnant women and young children
for a couple of days to minimise any risk to them. He/she will be
told not to use public transport or to go to public places to avoid
inadvertent contact with such individuals. |
After
the treatment the patient is NOT radioactive.
He/she may see other people straight away (although feeling unwell
may not wish to). |
LOJ
(February 2001)
- revised February 2003




 Uses of Nuclear Radiation
Uses of Nuclear Radiation
 Tracers in Industry
Tracers in Industry


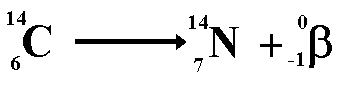
 Gamma rays kill bacteria. Therefore irradiating food or surgical instruments is a good way of ensuring they are sterile. The gamma rays penetrate packaging, so the food or instrument can be sealed and then sterilised so that re-contamination cannot occur.
Gamma rays kill bacteria. Therefore irradiating food or surgical instruments is a good way of ensuring they are sterile. The gamma rays penetrate packaging, so the food or instrument can be sealed and then sterilised so that re-contamination cannot occur.  Automatic control over the thickness of paper in paper mills can be obtained by passing beta radiation through the paper and monitoring the count rate. An isotope with a long half-life is used so that the count-rate hardly changes with time. Electrical circuitry is then set up to ensure that a constant rate is maintained. If the rate is too low the rollers automatically move closer to each other (making the paper thinner) and vice versa.
Automatic control over the thickness of paper in paper mills can be obtained by passing beta radiation through the paper and monitoring the count rate. An isotope with a long half-life is used so that the count-rate hardly changes with time. Electrical circuitry is then set up to ensure that a constant rate is maintained. If the rate is too low the rollers automatically move closer to each other (making the paper thinner) and vice versa.  The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions.
The Apollo Moon missions used a radioisotope thermal generator (RTG). The NASA designation for the devices that powered the Apollo Lunar Surface Experiments Package (ALSEP) for missions 12, 14, 15, 16, and 17 was SNAP-27 (Systems for Nuclear Auxiliary Power model number 27). The energy source for this device was a rod of plutonium-238 weighing approximately 2.5 kilograms and providing a thermal power of approximately 1250W. Plutonium-238 is a non-fissile isotope of plutonium that decays by alpha particle emission with essentially zero associated gamma emissions.  Smoke Detectors
Smoke Detectors  Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see
Many camping lantern mantles used to contain thorium (alpha emitter with a long half-life see  During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years.
During a fire, it's necessary to make sure that emergency exit signs remain illuminated, even if the power goes out. Some signs have a battery-powered light. Others have used tritium, a beta-emitting isotope of hydrogen, with a half-life of 12.3 years.  Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-gr
Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-gr Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document:
Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium. This resulted in illness. See the MIT research document: 
