    |
||||

Photoelectric Effect - evidence of light as a particle |
||||
|
Research into the Photoelectric Effect was initiated in 1887 by H. R. Hertz (the guy that the unit of frequency is named after!). He discovered that if ultra-violet light was shone on a spark gap in a vaccuum tube, it facilitated the passage of the spark. That led immediately to a series of investigations within Universities and the photoelectric effect was discovered. The failure of the classical theory of electromagnetic radiation to explain the photoelectric effect helped lead to the development of the quantum theory. According to classical physics theory, when light, thought to be composed of waves, strikes substances, the energy provided would depend on the intensity of that light. Bathing the metal with intense radiation (high amplitude light waves) would provide more energy per second to the metal than low intensity radiation. If classical physics had been correct it would mean that if you had intense red light bathing a metal strip you should be more likely to release electrons than if you bathed it with a weak higher frequency source. It would also be expected that there would be a time interval between the initial bathing with the light and the the first electron being ejected (time for the electron to absorb enough energy to escape). However was what was observed was not what had been expected. Light above a certain frequency, no matter how weak the source, could release photoelectrons instantly, whereas light of a lower frequency no matter how intense or how long the source was applied for had no effect...
The experiment
Using the classical Maxwell wave theory of light, the more intense the incident light the greater the energy with which the electrons should be ejected from the metal. That is, the average energy carried by an ejected (photoelectric) electron should increase with the intensity of the incident light. In fact, Lénard found
that this was not so. Rather, he found the energies of the emitted electrons
to be independent of the intensity of the incident radiation. So in classical physics, one would expect the current flow to be proportional to the total amount of light energy shone onto the metal - the strength of the beam of light and the time it was shining on the plate would both play a part. But this was NOT found to be the case. It was true that more light intensity meant more electrons were liberated and therefore more current was produced. However, whether the current flowed at all depended on the wavelength of the light - there was a sharp 'cut-off' wavelength above which no current at all flowed no matter how strong the beam or how long it shone for. In 1905 Einstein successfully explained the photoelectric effect within the context of the new physics of the time - quantum physics. In his scientific paper, he showed that it could be explained if light was thought of as being made of 'packets of energy' - 'quanta' of energy - photons. Each photon has a specific energy related to its wavelength, such that photons of short wavelength (blue light) carry more energy than long wavelength (red light) photons. E = hf = hc/λTo release an electron from a metal plate required a energy above a certain value. That energy was called the 'work function energy'. It was the energy required to do work against the structure of the metal that the electron was part of. This was a very important towards our current view that light has a dual nature - wave and particle. The photoelectric effect explanation earned Einstein the Nobel Prize, and first introduced the term 'photon' of light into our terminology. - so a photon is a quantum of electromagnetic energy with energy of hf - and only one photon can be absorbed by an electron at a time. This phenomenon could not be understood without the concept of light as being particle-like - a photon, a quantum amount of light energy for a particular frequency. If light were simple a wave-like phenomenon then increasing the intensity and thereby increasing the total energy falling on the surface would be expected to eventually provide enough energy to release electrons no matter what the frequency. Furthermore, in the classical picture one would expect the maximum energy of the emitted electrons to depend on the intensity of the light -- but it does not. So this is evidence that light behaves as if it were a particle. |
||||
 |
||||

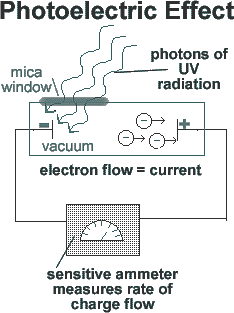 If a beam of light is pointed
at the negative end of a pair of charged plates enclosed in a vacuum,
a current flow is measured. Thus, the beam of light must be liberating
electrons from one metal plate, which are attracted to the other plate
by electrostatic forces, crossing the gap and completing the circuit.
This results in a current flow.
If a beam of light is pointed
at the negative end of a pair of charged plates enclosed in a vacuum,
a current flow is measured. Thus, the beam of light must be liberating
electrons from one metal plate, which are attracted to the other plate
by electrostatic forces, crossing the gap and completing the circuit.
This results in a current flow. 
