|
Much
of chemistry is devoted to the study of carbon based compounds because
carbon can form vast complex molecules. It is capable of doing this because
of its four valence electrons.
Silicon and germanium are also in group
IV and they too can form complex structures. All group IV elements can
be made to conduct electricity, even carbon, which is a non-metal.
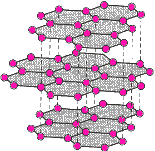 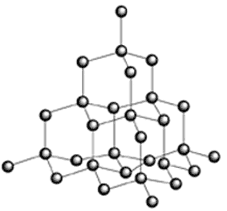 To
conduct you need weakly held electrons and the carbon structure of graphite
fits this descrpition in tems of weak pi-bonds (see diagram on the left) between sheets of hexagonally
covalently bonded sheets of carbon atoms - it is therefore a poor conductor....
but it IS a conductor! Wereas the covalent bonds in diamond (diagram on the right) make it a
non-conductor. To
conduct you need weakly held electrons and the carbon structure of graphite
fits this descrpition in tems of weak pi-bonds (see diagram on the left) between sheets of hexagonally
covalently bonded sheets of carbon atoms - it is therefore a poor conductor....
but it IS a conductor! Wereas the covalent bonds in diamond (diagram on the right) make it a
non-conductor.
Silicon is essentially non-metallic whereas
germanium is metalloid and tin and lead are metals.Germanium and silicon
ore called semi-conductors.
A semi-conductor has a higher conductivity
than an insulator and a lower conductivity than a conductor.
They have
a negative temperature co-efficient of resistance (i.e. as temperature
increases, resistance decreases. Compare this with metals which are the
opposite. At low temperatures some metals (tin and lead being two of them)
exhibit superconductivity: their resistance becomes virtually zero as
the temperature of absolute zero approaches). Pure elements that form
semiconductor crystals are termed INTRINSIC semiconductors as their
semiconductor nature is inherent.
Silicon is second
only to oxygen in weight percentage of the earth's crust (= 28%) whereas
germanium is relatively rare.
Within the pure germanium
or silicon crystal inter-atom bonds form a vast structure similar to that
of diamond (covalent tetrahedral 3-D formation of strong bonds).
NB
They cannot form a graphite-like structure; that is unique to carbon.
Carbon has two resistivities
(one for graphite, one for diamond). Taking diamond as the 'normal' structure,
electrical resistivity decreases down the group from and insulator (diamond)
through semiconductors (silicon and germanium) to metals (tin and lead).
(Graphite's resistivity comes between germanium and tin as it is a poor
conductor rather than a semiconductor).
Normal germanium crystal
In pure germanium
(or silicon) crystals the valency electrons bond with neighbouring atoms
to form a stable 3-D covalent lattice. High energy electrons within these
bonds can free themselves from the structure to form conduction electrons,
leaving positive 'holes' which could be viewed as positive charge carriers.
If heat energy is
given to the structure more electrons become free and more holes are formed
so the resistivity decreases (or conductivity increases) hence the negative
temperature co-efficient of resistance. Insulators also have a negative
temperature co-efficient of resistance. Above 100'C the lattice breaks
down completely and so temperature is an Important factor when using semiconductor
materials.
The conductivity of
semiconductor materials can be increased without raising temperature by
DOPING. Doping consists of introducing impuity atoms into the structure.
These Impurities are usually from groups III or V.
Those from group V have a surplus valence electron when they are bonded
within the group IV element's crystal structure. (NB they haven't an overall
charge, Just an un-bonded electron). So if phosphorus (P), Arsenic (As)
or Antimony (SB) atoms are introduced into the lattice they are able to
donate electrons to be conduction electrons and increase the conductivity
of the crystal (they do not make it negative!!) they are therefore called
DONOR atoms. The semiconductor has gained negative charge carriers and
is therefore called an n-type semiconductor.
Those from group III have a missing valence electron when they are bonded
within the group IV element's crystal structure. (NB they haven't an overall
charge, they are Just an electron short to fit in with a quadravalent
structure). So if boron (B), Aluminium (Al), Gallium (Ga) or Indium (In)
atoms are introduced into the lattice they produce holes in the lattice.
These holes mop up stay conduction electrons that left their original
position because of surplus energy. Thus holes 'move' like positive charge
carriers because holes move in the opposite direction of electrons. A
small amount of dopant causes a very large number of holes compared to
the number of free electrons in the pure or 'intrinsic1 semiconductor.
Thus the charge carriers are increased, but they are positive charge carriers
and the conductivity of the crystal increases (they do not make it positive!!).
They accept electrons into the holes and they are therefore called ACCEPTOR
atoms. The semiconductor has gained positive charge carriers and is therefore
called a p-type semiconductor.
The
charges balance in a semiconductor - it does NOT have a net charge!
|