|
The half-life of radioactive zinc is 50 hours. Find the percentage
loss of activity which occurs for a sample each hour.
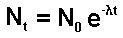
Nt = XN0 (where X = factor of radioisotope
remaining undecayed)
t = 1 hour
half life = 50 hours
l = ln2/(half
life) = 0.693/50 hours-1 = 0.01386 hours-1
X = e-lt
ln X = -lt
ln X = - 0.01386 x 1 = -0.01386
X = 0.986
Therefore the percentage undecayed = 98.6%
and the percentage decayed = 1.4%
loss of activity after 1 hour = 1.4%
|