|
The half-life of Pb 210 is 20 years. Find the initial number of disintegrations
per second for 1.0g of this isotope of lead.
210g lead contains NA (6.02 x 1023) atoms
therefore 1 g lead contains 6.02 x 1023/210 atoms
(LEAVE IT IN THIS FORM… IT IS NOT A GOOD IDEA TO KEEP ON WORKING
OUT SUBSTAGES)
N0 = 6.02 x 1023/210
dN/dt = ?
half life
= 20 years
= 20 x 365.25 days
= 20 x 365.25 x 24 hours
= 20 x 365.25 x 24 x 60 hours
= 20 x 365.25 x 24 x 60 x 60 seconds
= 6.31 x 108 seconds
l = - ln2/half
life = - 0.693/(6.31 x 108)
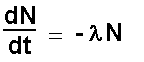
= - (- 0.693 /(6.31 x 108)) x (6.02 x 1023/210)
= (0.693 x 6.02 x 1023 )/ (210 x 6.31 x 108)
= 3.1 x 1012 s-1
|