|
The half-life of cobalt 61 is 100 minutes. How long does it take for
the activity of the sample to fall to 80% of its initial value?
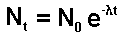
Nt = 0.8N0
t = ?
half life = 100 minutes
l = ln2/(half
life) = 0.693/100 minutes-1 = 0.00693 minutes-1
0.8 = e-lt
Take logs
ln 0.8 = -lt
Substitute for l
ln 0.8 = - 0.00693 x t
Look up the log
-0.223 = - 0.00693 x t
t = 32 minutes
|