|
|||||||||||||||||||||||||
 Choice
of radionuclide for an imaging study Choice
of radionuclide for an imaging study |
|||||||||||||||||||||||||
|
The radioisotope must be suitable
for labelling a chemical compound that performs a function in the organ
to be examined. Isotopic labelling involves replacing an identical atom
with that of a radioactive isotope and this is ideal because it will
not cause a pharmacological response (or even be toxic). If non-isotopic
labelling is employed it is necessary to introduce not only the
tracer but also non-radioactive compounds of the same type to 'bulk'
the solution as such a tiny amount of radioactive isotope is employed
- pharmacological response is then important.
Type of radiation emitted is very important, the best is a pure gamma emitter. Gamma emission often follows closely on a or b emission making the isotope unsuitable. Also the nucleus should not be likely to emit a or b after emission of the g ray either, so decay to the next atom in the chain should involve a long half-life. The energy of the g-ray emitted should be suitable for viewing with a gamma-camera (energy range 100 - 400 keV gives optimum detection, is easy to collimate and has low attenuation in the body).
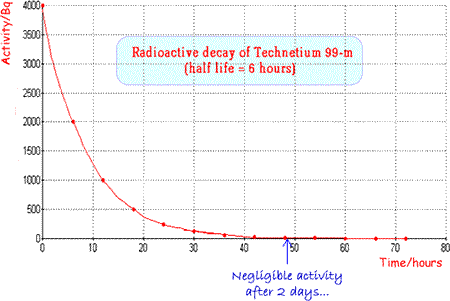
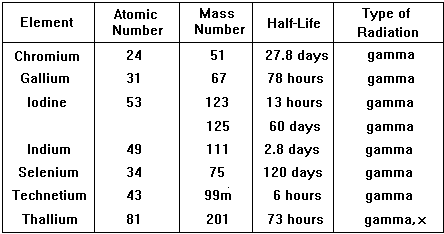
The physical half-life should be ideally a few hours so that activity is high enough for the investigation to be carried out but then rapidly drops, enabling the patient to quickly resume normal life. The patient also avoids receiving a high dose of radiation. Examples of specific radionuclides used in clinical measurements
An ideal and very commonly used tracer is technicium 99m |
|||||||||||||||||||||||||
 |
|||||||||||||||||||||||||