|
There
are four types of nuclear
radiation:
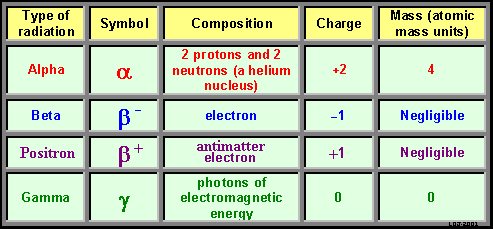
 Alpha
particles: Alpha
particles:
These are matter
particles made up of clusters of 2 neutrons and 2 protons.
Basically they are heium nuclei - it is believed that protons and
neutrons within the nucleus are arranged in clusters like this as they
are so stable.
They are therefore emitted as a 'package'
 Beta
Particles: Beta
Particles:
These are matter particles that are identical to the electrons
that are found orbiting the nucleus.
They only differ in where thgey originate - they emanate from the nucleus
(which doesn't contain electrons!).
All experiments with beta particles
show them to be identical to orbital electrons - mass and charge are
identical. They are produced when a neutron breaks down and becomes
a proton.
 Gamma
Photons: packets of high energy electromagnetic
radiation... pure energy, no matter! High
frequency and high energy. The only sort of electromagnetic radiation
that comes from the nucleus is the gamma ray. Gamma
Photons: packets of high energy electromagnetic
radiation... pure energy, no matter! High
frequency and high energy. The only sort of electromagnetic radiation
that comes from the nucleus is the gamma ray.
 Antimatter
Beta Particles (Positrons): Antimatter
Beta Particles (Positrons):
They are antimatter yet they emanate from the matter
in the nucleus!
These are only given
out by the nuclei of nuclides that have too many protons to be stable.
These have only been discovered since we have been able to artificially
'make' isotopes that did not occur naturally on Earth during the last
century. Some of these decayed to produce these antimatter particles.
If a positron meets up with an electron they annihilate
each other. and gamma rays are produced (but that is only tackled at
Advanced level!).
|




 Types
of Nuclear Radiation
Types
of Nuclear Radiation 



