|
||||
 Radioactive
rocks Radioactive
rocks |
||||
|
Uranium-238 has a very long half-life so deposits of it are still quite abundant around the world. It is an alpha particle emitter so it is not dangerous to living things when it is outside the body. However, tiny particles of rock are dissolved in water or broken off by the wind during the process of erosion. These tiny alpha emitters can be ingested (drank or eaten) or inhaled (breathed into the lungs). Alpha emitters inside the body are very
dangerous.
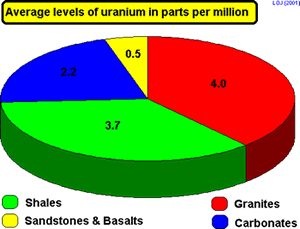
Igneous Rock: Granite
Sedimentary rock: limestone or sandstone
Metamorphic rock
If a rock contains
a vein of uranium ore the concentration of uranium can be as great as
5000 parts per million but in general the levels are much lower.

Radioisotopes decay
to give other radioisotopes in a radioactive
decay series. Part of the decay series for uranium is the gas radon.
This means that rocks with uranium content are not only potentially
hazardous when tiny grains are transported by erosion but also from
the radon gas they emit. (See Radon Gas) Age of rocksThe most common and accepted method of establishing the age of rocks is based on the natural radioactivity of certain minerals found in rocks. Once the rate of radioactive decay of any particular isotope has been found, the age of a specimen can be worked out from the ratio of the remaining isotope to its decay product. The older it is the more decay product will be present. In recent geology carbon-dating can be used to date specimens as old as 35,000 years. |
||||
 |
||||