|
If the equilibrium concentration of carbon 14 in living plants gives
16 disintegrations per minute per gramme of carbon, estimate the age
of a piece of timber if 2.0g of carbon prepared from it gives 15 disintegrations
per minute. (The half-life of carbon 14 is 5.57 x 103 years.)
14g carbon 14 contains NA atoms
therefore a 2g sample contains (6.02 x 1023) / 7 atoms
N = (6.02 x 1023 ) / 7
half-life = 5.57 x 103 years
= 5.57 x 103 x 365.25
x 24 = 4.88 x 107 hours
= 2.93 x 109 minutes
l = ln2/half-life
= 0.693 / 2.93 x 109 = 2.37 x 10-10 minutes
-1
dN/dt = -lN
Age is zero when dN/dt = 16 min-1 g-1
Age is t when dN/dt = 15 min-1 for a 2g sample = 7.5 min-1
g-1
the ratio of dN/dt to N is constant therefore 16/N0 = 7.5/Nt
and Nt /N0 = 7.5/16
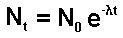
So,
e-lt
= 7.5/16
Taking logs
-lt
= ln (7.5/16)
t = -ln(7.5/16)/l
t = 0.758/(2.37 x 10-10)
=3.2 x 109 minutes
= 6080 years
= 6100 years (2 sig figs)
|