|
|||||||||||||||||||||||||

Nuclear Power - fuel rods |
|||||||||||||||||||||||||
|
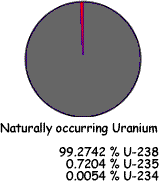
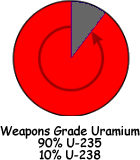
In order to be a nuclear fuel isotope the nucleus has to be capable of fission and form part of a critical mass of such nuclei. Of all the potential nuclear fuels found in nature, only Uranium-235 is suitable, but any naturally occurring sample of uranium would not have enough U-235 within it to form a critical mass. It must therefore be 'enriched' to increases the concentration of Uranium-235.
Natural uranium is made up of:
Why is the fuel made up of rods rather than being a solid single piece of fuel? To maximise efficiency the moderator needs to be interspersed between the fuel. Neutrons need to pass through a moderator to slow them (in order to cause further fissions or prevent U-238 absorbing them) - this can be inserted between fuel rods. If a neutron passes out of the fuel rod it is unlikely to re-enter it, but there is a possibility that it may enter another one. It also makes it easier to replace the fuel in stages. Why Spent Fuel Rods are More Dangerous than Unused Fuel Rods Fission fragments are in general neutron heavy and therefore radioactive or unstable emitting beta and gamma radiation Some fission fragments have short half-lives or high activities whereas the Uranium has a long half life and a relatively low activity compared to the fission products. Therefore the activity of spent fuel rods is much higher than ones that have not been inserted in a reactor. Why, after a period of use, the fuel rods in a nuclear reactor become less effective for power production Power production depends upon the number of fissions in unit time. After a period of time the amount of (fissionable) uranium (235) in the fuel decreases because they have undergone fission. Two fission fragments are produced for each completed fission reaction and they can absorb neutrons preventing further fission.
|
|||||||||||||||||||||||||
 |
|||||||||||||||||||||||||