|
|||||||||||||||||||||||

Uranium |
|||||||||||||||||||||||
|
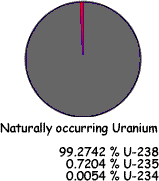
Natural uranium is made up of:
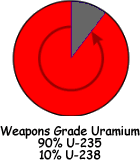
Depleted Uranium: The byproduct of enrichment is called depleted uranium or DU. It is mainly made up of of the isotope uranium-238 (U-238). It contains less than one third as much U-235 and U-234 as natural uranium. Therefore the external radiation dose from DU is about 60 percent of that from the same mass of natural uranium. Another less common source of DU is reprocessed spent nuclear reactor fuel rods. This can be distinguished from DU produced as a byproduct of uranium enrichment by the presence of U-236 (half life 2.342×107 years). This is not naturally found on Earth despite its long half life. It is the isotope that is produced in nuclear reactors by firing neutrons at U235. It very readily undergoes fission - but some atoms of it remain that have not undergone fission and can be used to identify spent fuel rods. DU is used for its very high density of 19.1 g/cm3.
Depleted uranium munitions are controversial because of unanswered questions about potential long-term health effects. DU is less toxic than other heavy metals such as arsenic and mercury, and is only very weakly radioactive because of its long half-life.. While all radiation exposure has risks, no conclusive epidemiological data have correlated DU exposure to specific human health effects such as cancer (there are always so many other factors that can trigger it - and the effect takes decades not months or years) . However, the UK government has attributed birth defect claims from a 1991 Gulf War combat veteran to DU poisoning, and studies using cultured cells and laboratory rodents continue to suggest the possibility of leukemogenic, genetic, reproductive, and neurological effects from chronic exposure. Data is still being collected on its effects. See this article in Medical News Today.
|
|||||||||||||||||||||||
 |
|||||||||||||||||||||||