|
To investigate the relationship between molecular kinetic energy and temperature
From the kinetic theory of gases:
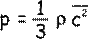
Therefore let us multiply both sides by V to get an expression for any volume V of gas:
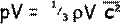
Now
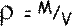
So,
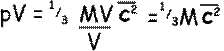
Where M is the total mass of the gas of volume V.
Now, M = Nm ( where m is the mass of one particle in the gas and N is the number of particles in the gas)
So
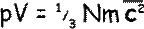
A bit of mathematical magic can make that last part of the equation represent kinetic energy. To do that we need a ½ factor before the m… to make it fair we have to put a factor of 2 in front of the N:
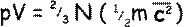
Now the Equation of State or Ideal Gas Equation (which you should know by heart!) is
pV = nRT
where
R = the universal molar gas constant,
T = the temperature in kelvin, and
n = number of moles of gas
We can therefore put:
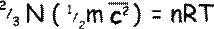
Rearranging the equation we get that:
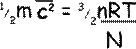
Now, as N/n is the number of molecules per mole, i.e. NA, the Avogadro constant, so we can get rid of the factors that require us to know the details of the gas and replace them with a constant.
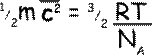
Both R and N A are universal constants, and therefore so also is R/N A; it is called Boltzmann's constant, ‘k’ and it is the gas constant per particle. The left-hand term is the average translational kinetic energy of a single molecule, and therefore
Average translational kinetic energy of a particle of gas = 3/2kT
 Things to remember! Things to remember!
- ‘T’ must be in kelvin – remember to convert!
- The Ek is in joules and is for one average particle – the question often asks you for the energy of a number of moles of gas.
|