|
graphic from
http://www.nylcv.org
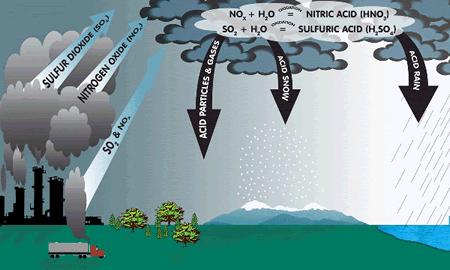
Coal (and to a lesser degree oil) contains traces of sulphur.
When coal or oil is burnt, the sulphur oxidizes (reacts with oxygen) to form sulphur dioxide gas.
sulphur + oxygen  sulphur dioxide. sulphur dioxide.
S(s) + O2(g)  SO2(g) SO2(g)
Sulphur dioxide gas is acidic, poisonous, dissolves very well in water and smells like bad eggs!
It reacts with water and oxygen in the atmosphere to form a dilute solution of hydrogen sulphate (sulphuric acid), it is the main pollutant in acid rain.
Natural rain is slightly acidic due to dissolved carbon dioxide but natural rain has a pH of about 5.5, whereas acid rain has a pH of about 4.
The second most important pollutant in acid rain is nitric acid - NO2 emissions from factories add that to the atmosphere.
 Acid rain kills trees. It runs into rivers and gathers in lakes and eventually the lakes become too acidic and plants and fish begin to die. It also reacts with limestone and damages limestone buildings. Acid rain kills trees. It runs into rivers and gathers in lakes and eventually the lakes become too acidic and plants and fish begin to die. It also reacts with limestone and damages limestone buildings.
Powdered limestone or slaked lime (alkaline) can be added to soils or lakes to make them less acidic but it would be better if we could avoid or reduce pollutant gas emissions in the first place!
|