|
Coulomb's law, developed in the 1780s by French physicist Charles Augustin de Coulomb, may be stated as follows:
The Coulomb Force is the electric force (that acts on charges) is given by the following
equation:

where 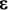 o
is the permitivitty of free
space, o
is the permitivitty of free
space,
Q1 and Q2 are the charges and
r is the
distance they are apart
The Coulomb force is a force - so it is a vector!
(a positive
result indicates a repulsive force (two negative charges or two positives
will result in that), whereas a negative result indicates an attractive
result (one charge of each type will result in that))
The gravitational force
(that acts on masses) is given my the following equation:
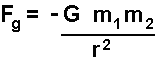
(the negative sign shows that the force is always attractive)
where G is
the gravitational constant, m1 and m2 are the
masses and r is the distance they are apart.
Can you see the similarity? The examiners will expect you to!
Consider two electrons one
metre apart:
- mass of
the electron me = 9.11 x 10-31 kg
- charge on the electron Qe = 1.60 x 10-19 C
- G = 6.67 x 10-11 m3 kg-1 s-2
 o
= 8.85 x 10-12 F m-1 o
= 8.85 x 10-12 F m-1
The gravitational force acting
on the two electrons is 8.3 X 10 -61 N
The coulomb force acting on the two electrons is 2.3 x 10-28 N
Therefore the coulomb force
is 1032 times bigger than the gravitational force (100,000,000,000,000,000,000,000,000,000,000
times bigger).... that's why we have to think of charges as being in
a different dimension to us.... they experience the 'hills and dales of the electric
field' much more strongly than the 'ups and downs' of the gravitational field.
LOJ MARCH
2002
|