|
|||||

Depletion of the Ozone Layer |
|||||
|
The Earth's ozone layer protects all life from the sun's harmful ultraviolet radiation (UVB), but human activities have damaged this shield. Less protection from ultraviolet light will, over time, lead to higher skin cancer and cataract rates and crop damage. The Earth's atmosphere is divided into several layers. The lowest region, the troposphere, extends from the Earth's surface up to about 10 kilometers (km) in altitude. Virtually all human activities occur in the troposphere. Mt. Everest, the tallest mountain on the planet, is only about 9 km high. The next layer, the stratosphere, continues from 10 km to about 50 km. Most atmospheric ozone is concentrated in a layer in the stratosphere, about 15-30 kilometers above the Earth's surface. 'Normal' oxygen, which we breathe, has two oxygen atoms, double covalently bonded together and is colourless and odourless. It has the formula O2. What is ozone?
Ozone production in the atmosphere
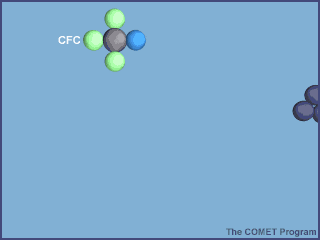
Human intervention disturbed the equilibrium and incresed UV levels reaching the surfaceThis was the situation until the past several decades when our production of certain chemicals (mainly chemicals used in the cooling systems in refrigerators and as propellants in aerosol cannisters - CFCs) upset that balance and shifted the equilibrium so that the ozone layer that protects the Earth from harmful ultraviolet light became thinner - effectively creating a 'hole' over the poles.
Ozone-Depleting Substances (ODS)ODS include chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), halons, methyl bromide, carbon tetrachloride, hydrobromofluorocarbons, chlorobromomethane, and methyl chloroform. ODS are generally very stable in the troposphere and only degrade under intense ultraviolet light in the stratosphere. It is the large increases in stratospheric ODS that has upset the Earth's ozone cycle equilibrium by removing ozone faster than natural ozone creation reactions can work at. Therefore, ozone levels fell dramatically. Over the past couple of decades we have worked hard to stop these chemicals from entering the atmosphere and ozone depletion is less of a problem, but the ozone layer is still thinner than it used to be and will take a long time to recover from the damage we have done to it. In 1987, the United Nations established the Montreal Protocol to regulate the quantities of these gases in the atmosphere. The Montreal Protocol was signed by 197 countries, and is the only UN treaty in history to achieve universal ratification. Over time, the protocol has been updated to include more substances that could cause harm to the ozone layer and the climate. For example, in 2016, hydrofluorocarbons were added to the list of controlled substances, because these gases were identified as potent greenhouse gases. The treaty is considered to be one of the most successful protocols to tackle an environmental challenge caused by humans. Back in the autumn of 2006 the hole in the ozone layer was the largest it has ever been at 29.6 million square miles, larger than Antarctica itself! This was due to a number of factors, natural conditions as well as man-made. Since that time ozone levels first remained static and then the hole started to decrease in size but we have a long way to go. Most scientists agree that the ozone layer will recover, but signs of recovery are yet to be seen and full recovery will not take place until the ned of the 21st century. Why is a 'hole in the ozone layer' a problem?Since ozone filters out harmful UVB radiation, less ozone means higher UVB levels at the Earth's surface. The more depletion, the larger the increase in incoming UVB. UVB has been linked to skin cancer, cataracts, damage to materials like plastics, and harm to certain crops and marine organisms. Although some UVB reaches the surface even without ozone depletion, its harmful effects will increase as a result of this problem. How does ozone depletion occur?
The ozone depletion process begins when CFCs and other ozone-depleting substances (ODS) are emitted into the atmosphere. Winds efficiently mix the troposphere and evenly distribute the gases. CFCs are extremely stable, and they do not dissolve in rain. After a period of several years, ODS molecules reach the stratosphere, about 10 kilometers above the Earth's surface . Strong UV light breaks apart the ODS molecule. CFCs, HCFCs, carbon tetrachloride, methyl chloroform, and other gases release chlorine atoms, and halons and methyl bromide release bromine atoms. It is these atoms that actually destroy ozone, not the intact ODS molecule.
The information and graphics on this page were taken from http://www.epa.gov with kind permission. For stories about ozone layer depletion in the news - click here |
|||||
 |
|||||

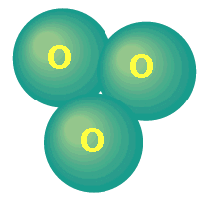
 The name ozone is derived from a Greek word
meaning "to smell".
The name ozone is derived from a Greek word
meaning "to smell". 


 It is estimated that one chlorine atom can
destroy over 100,000 ozone molecules before it is finally being removed
from the stratosphere!!
It is estimated that one chlorine atom can
destroy over 100,000 ozone molecules before it is finally being removed
from the stratosphere!!