|
Electrons exist in
energy states within the atom (called orbitals by chemists).
Generally,
the further away from the nucleus these states are, the higher the potential
energy of the electron in that state. When an electron is 'free' of the
nucleus' influence it is said to have zero potential energy - a zero energy
state. When it is closer to the nucleus it has less potential energy (needs
energy input to escape the influence). It is therefore at a negative energy
level
We can put information about these energy states into a diagram
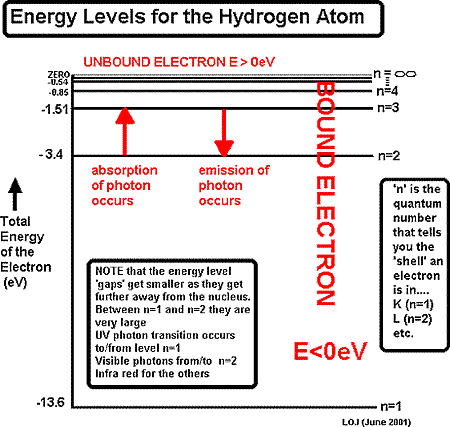
When an electron jumps between energy levels
of an atom it either absorbs energy for the transition or emits a photon when reducing its potential energy.
It can obtain the energy required for 'promotion' to a higher energy state by several means:
 photon absorption - absorbing a photon of exactly the correct energy needed for a 'jump'. photon absorption - absorbing a photon of exactly the correct energy needed for a 'jump'.
 obtaining energy by colliding with a free electron. It will take just the amount of energy it needs for the jump from the free electron's kinetic energy. obtaining energy by colliding with a free electron. It will take just the amount of energy it needs for the jump from the free electron's kinetic energy.
 an electron can obtain energy from an electrical supply. W = QV, so an electron, having a charge 'e' will obtain energy of XeV if placed in an potential difference of X volts. an electron can obtain energy from an electrical supply. W = QV, so an electron, having a charge 'e' will obtain energy of XeV if placed in an potential difference of X volts.
When absorbing or emitting a photon, the size of that photon's
energy depends on the energy gap between the levels. This is linked to
its frequency by the equation
E = hf
Where
E is the energy of the photon absorbed
or emitted -the energy interval between the energy levels
(in joule)
h = Planck constant
f = frequency of the photon of electromagnetic
radiation (linked to its wavelength by c = f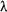 ) )
ΔE = hf =
hc/λ
Exciting the electrons
This can be done by bathing the gas sample
in electromagnetic energy of a wide continuous spectrum (usually of visible light - but it is the same principle for other sections of the electromagnetic spectrum - only then you wouldn't 'see' the result - you would need to use a detector).
The electrons
absorb the photons they need to make transitions to higher energy levels
and then give them back out again when they return to the ground state.
They only remain in an excited state for less than a microsecond and are
therefore constantly absorbing/emitting photons from the electromagnetic
source.
Or it can be done by bombarding the atoms with electrons. They will then share their kinetic energy with the orbital electrons, causing them to be promoted.
When they return to the ground state the electrons can do so by any path - jumping down any number of energy levels to get to the ground state. You may be asked to calculate the frequency or wavelength of the emitted photons.
You will use the equation:
E = hf = hc/λ
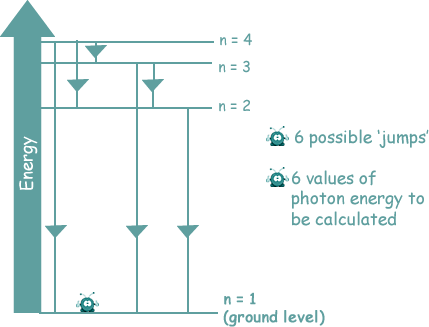
Atomic Spectra
There are two type of atomic spectra. The graphic below shows the visible spectra for hydrogen.
You can see that the lines are in the same place on both spectra - because they correspond to the same energy jumps.
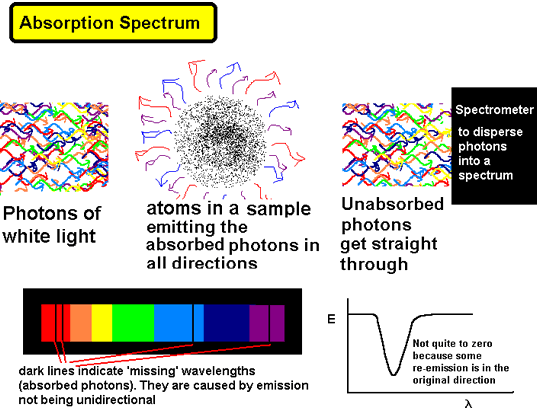
Photons that do not have energies that correspond
to the 'gaps' between energy levels for the electrons are not absorbed.
They go through the gas onto the photographic plate (or other form of
detector).
When they give out the photons they have
absorbed they do so in random directions therefore only a tiny fraction
of them are emitted in the direction they would have gone had they not
been absorbed in the frst place. This results in a lower intensity of
electromagnetic radiation of the wavelength that has been absorbed by
the sample shining onto a photographic plate. We therefore get an absorption
spectrum
Click here
for an interactive activity page on absoption spectra.
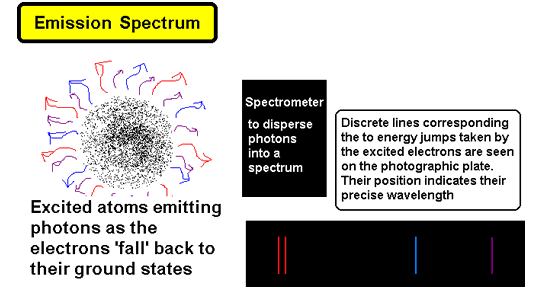
Another way of exciting the electrons is
electrically. By putting the gas in a discharge tube we can 'zap' the
atoms and therefore their electrons with electrical energy. This promotes
electrons from the ground state to excited states and then as they 'jump
down' they emit the characteristic photons of the jumps within the electron
orbital of the atom. These can be collected by a dispersion system such
as a spectrometer (or prism) which will disperse them into wavelengths....
missing wavelengths will not appear on the film, gaps will be left. The
only coloured lines will be those that correspond to the wavelengths of
the photons that 'fit' the energy jumps between orbitals. The spectrum
we see is called an emission spectrum.

 Click here
for an interactive activity page on emission spectra. Click here
for an interactive activity page on emission spectra.
We can look at these spectra using a spectrometer. This
is an instrument that used either prisms
to disperse the light by refraction
or diffraction gratings
to disperse the light by diffraction
 Check out this
link and look at the spectra of various gases in dischage tubes Check out this
link and look at the spectra of various gases in dischage tubes
 Click here for details as to how fluorescent
tubes work. Click here for details as to how fluorescent
tubes work.
 Click here to see an interactive application from Colorado Uni that allows you to see what happens in various models of the hydrogen atom. Click here to see an interactive application from Colorado Uni that allows you to see what happens in various models of the hydrogen atom.
 Click here to see how a discharge tube works. Click here to see how a discharge tube works.
LOJ
June 2001
 |
Ready for some questions?
Click on the icon - questions and answers at A level standard await you!
For more topics that have practice questions associated with them see the top menu bar - examination preparation
|
|















