|
||||

More about 'electron orbitals' |
||||
|
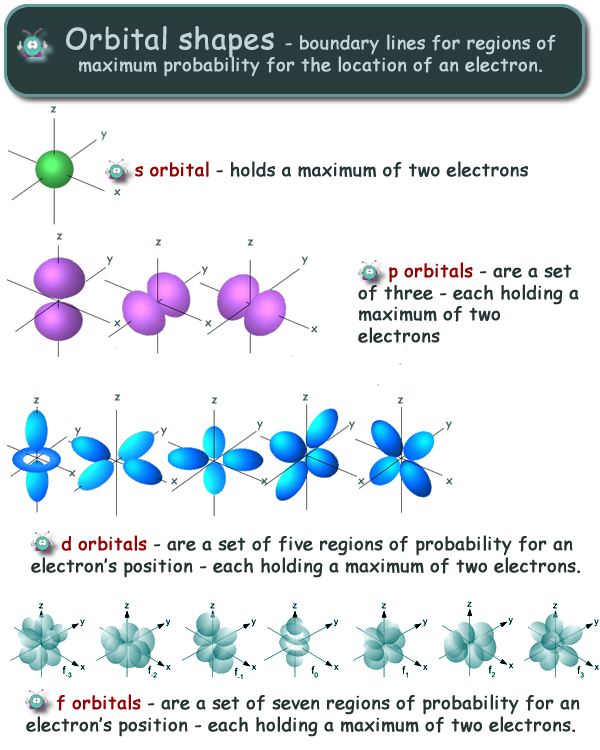
At A level each shell is given a quantum number 'n'. The shell nearest to the nucleus is given the quantum number n = 1. At GCSE you will have learnt that the inner shell only holds 2 electrons. This is because at that energy level there is only one s subshell. The next shell (n = 2) has an s orbital and a set of p orbitals - so it can hold eight electrons. The third shell has an s orbital, a set of p orbitals and d orbitals - it therefore holds eighteen electrons. Each subshell has a specific number of orbitals: s = 1 orbital, p = 3 orbitals, d = 5 orbitals, and f = 7 orbitals. One orbital can contain a maximum number of two electrons.Electron orbitals are simply a boundary line around the region of space where there is a maximum probabilty of locating an electron. Exactly how they move within that space is unknown, and many prefer to think of them 'orbiting' the nucleus - but this has been shown to be untrue. There are four types of orbital - s, p, d and f and their shapes might interest you....
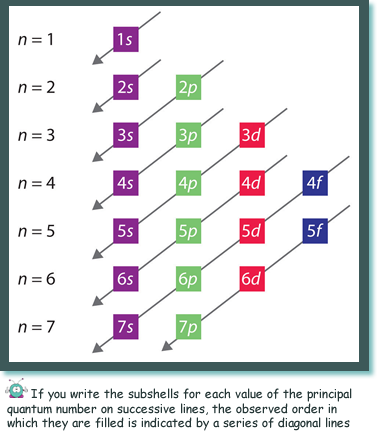
The order in which the shells are filled is related to the order of energies of the shells. Those at lower energy fill first. (See the diagram on the right). That information is more of interest to the chemists that the physicists but how the electrons behave is a fascinating subject, isn't it?
|
||||
 |
||||

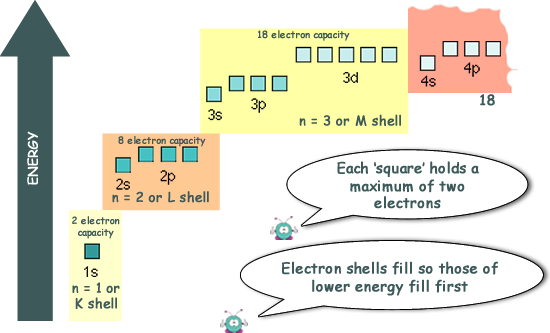 At GCSE you will have learned that the electrons occupy 'shells' - the inner one only holding 2 electrons, then one holding 8 electrons, then 18 etc.
At GCSE you will have learned that the electrons occupy 'shells' - the inner one only holding 2 electrons, then one holding 8 electrons, then 18 etc.