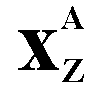|
|
(Greek letter 'a' - alpha) |
(Greek letter 'a' - alpha) |
(Chemical symbol for helium) 4 is the nucleon number or mass number (sum of protons and neutrons in the nucleus) 2 is the proton number or atomic number - the charge on the particle |
|
|
(Greek letter 'b' - beta) |
(Greek letter 'b' - beta) |
('e' stands for electron) we have a -1 because its charge is -1 and a zero for mass because it's mass is negligible |
|
|
|
||
|
|
(Greek letter 'b' - beta) |
(Greek letter 'b' - beta) |
('e' stands for electron) we have a +1 because its charge is +1 and a zero for mass because it's mass is negligible |